NCERT Solutions for Class 9 Science Chapter 1
The chapter “Matter in Our Surroundings” from your science textbook dives into the fundamental building blocks of everything around you! Here’s a quick summary of Matter in Our Surroundings:
What is Matter?
Matter is anything that occupies space and has mass. This means you can hold it, and it takes up space. From the chair you’re sitting on to the air you breathe, it’s all matter!
States of Matter:
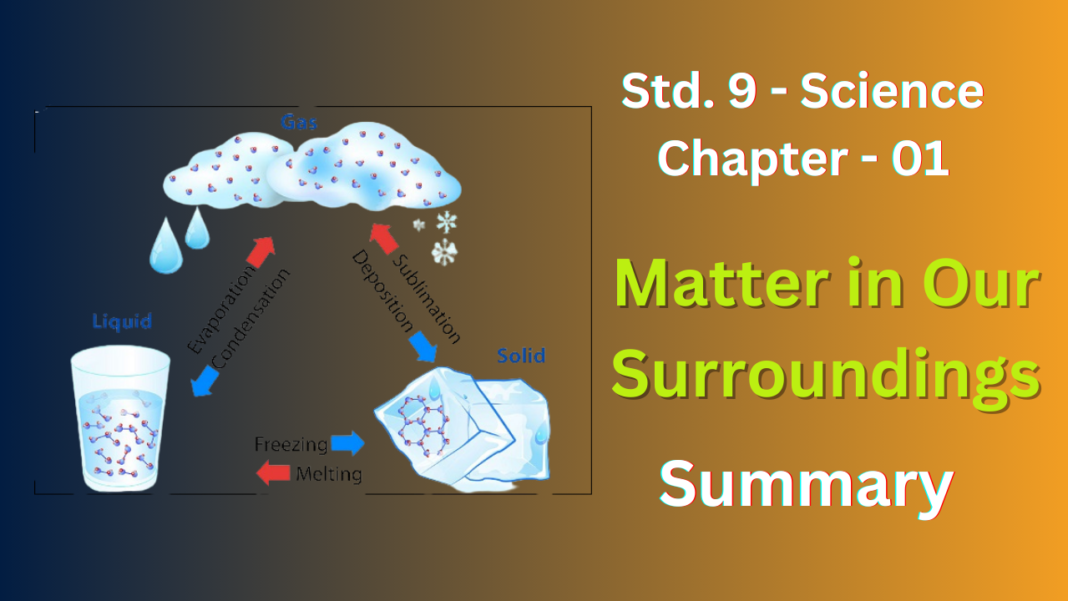
Matter can exist in three main states:
- Solids: These have a fixed shape and size. The particles (tiny building blocks) in solids are tightly packed with very little space between them. Think of a rock – it keeps its shape and you can’t easily squish it.
- Liquids: Liquids have a definite volume (amount of space they occupy) but can take the shape of their container. The particles in liquids are closer together than in gases but still have some space to move around. Water is a good example – it fills the cup you pour it into but doesn’t have a fixed shape on its own.
- Gases: Gases don’t have a fixed shape or volume and will spread out to fill any container. The particles in gases are far apart and move around very freely. Air is a kind of gas, that’s why it fills up a balloon completely.
Properties of Matter:
The chapter Matter in Our Surroundings also explores some properties of matter that help us understand how it behaves:
- Density: This is how much mass is packed into a certain volume. Think of a heavy bowling ball compared to a beach ball – the bowling ball has more density because it has more mass in the same amount of space.
- Compressibility: This refers to how easily matter can be squeezed into a smaller volume. Gases and liquids can be compressed to some extent, while solids are generally difficult to compress.
- Fluidity: This is the ability of matter to flow. Liquids and gases can flow, while solids generally don’t.
By understanding matter and its different states and properties, you gain a foundation for exploring the fascinating world of science!
NCERT Solutions for Class 9 Science Chapter 1
Q1. Which of the following matter? Chair, air, love, smell, hate, almonds, thought, cold, cold-drink, smell of perfume.
Ans: Out of the options you listed, only these are considered matter:
- Chair
- Air
- Almonds
- Cold-drink
- Smell of perfume
Q2. Give reasons for the following observation: The smell of hot sizzling food reaches you several meters away, but to get the smell from cold food you have to go close.
Ans: Hot food smells travel farther because heat makes odor molecules move faster (diffusion) and vaporize more readily, spreading the scent in the air. Cold food has less movement and vaporization, making the smell weaker and requiring you to be closer.
Q3. A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Ans: Divers cut through water due to intermolecular spaces. Water molecules have space between them, allowing the diver to move through.
Q4. What are the characteristics of the particles of matter?
Ans: There are three key characteristics of the particles of matter:
- Very small: These particles are incredibly tiny, so small we can’t see them with our naked eye.
- Have spaces between them: Even though matter seems solid, the particles themselves don’t touch. There’s empty space between them.
- Constantly moving: These particles are always in motion, although the speed of movement varies depending on the state of matter (solid, liquid, or gas).
Q5. Give reasons
(a) A gas fills completely the vessel in which it is kept.
(b) A gas exerts pressure on the walls of the container.
(c) A wooden table should be called a solid.
(d) We can easily move our hands in air but to do the same through a solid block of wood we need a karate expert.
Ans:
a) Gas fills the vessel: Gas particles have weak attraction and move freely, filling all available space.
(b) Gas exerts pressure: Gas particles constantly collide with the container walls, creating outward force (pressure).
(c) Wooden table is a solid: Strong forces of attraction between particles in wood give it a fixed shape and volume.
(d) Easy to move in air, hard in wood: Weak attraction and space between gas particles allow easy movement. Strong attraction and tight packing in solids make movement difficult.
Q6. Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
Ans: Ice floats because water molecules arrange differently when freezing, creating more space and lower density in ice compared to liquid water.
Q7. Convert the following temperature to Celsius scale:
(a) 300 K
(b) 573 K
Ans: (a) To convert 300 Kelvin (K) to Celsius (°C), we can use the following formula:
°C = K – 273.15
Therefore, 300 K = 300 K – 273.15 = 26.85 °C (approx.)
(b) Following the same formula for 573 K:
°C = 573 K – 273.15 = 300 °C (approx.)
Q8. What is the physical state of water at: (a) 250°C (b) 100°C
Ans: (a) At 250°C,
(b) At 100°C
Q9. For any substance, why does the temperature remain constant during the change of state?
Ans: During a state change, the supplied heat isn’t increasing temperature. Instead, it’s being used to overcome the forces between particles, allowing them to rearrange for the new state. This hidden heat is called latent heat, keeping the temperature constant.l
Q10. Suggest a method to liquefy atmospheric gases?
Ans: To liquefy atmospheric gases, we cool them (reduce particle movement) and compress them (force them closer together). This can be achieved through Joule-Thomson throttling, where compression and expansion cycles cool and condense the gas.
Q11.Why does a desert cooler cool better on a hot dry day?
Ans: Desert coolers cool better on hot dry days because:
- Dry air: Less moisture in the air (low humidity) means faster evaporation of water from the cooler.
- Hot air: Hotter air provides more energy to turn water from the cooler into vapour, speeding up evaporation.
Q12. How does the water kept in an earthen pot (matka) become cool during summer?
Ans: Earthen pots cool water through evaporation. Tiny pores in the pot let water seep out. As this water evaporates from the surface, it takes heat from the remaining water inside the pot, making it cooler.
Q13. Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Ans: Acetone, petrol, and perfume cool your palm because they are volatile liquids. As they evaporate, they absorb heat from your skin, making it feel cold.
Q14. Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
Ans: Saucer isn’t better for hot drinks. While it has a larger surface area for faster cooling, a cup is better for:
- Insulation: Keeps the drink warm for comfortable sipping.
- Sipping control: Cup design allows for easier and cleaner drinking.
Q15. What type of clothes should we wear in summer?
Ans: During summer, you’ll want to wear clothes that help you stay cool. Here’s the ideal combination:
- Fabric: Opt for natural, breathable fabrics like cotton. Cotton absorbs sweat and allows for evaporation, which cools your body down.
- Colour: Lighter colours like white or pastels are better. Dark colours absorb more heat, making you feel hotter.
- Style: Loose-fitting clothes allow for better air circulation around your body, keeping you cooler.
Q16. Convert the following temperatures to the Celsius scale. (a) 293 K (b) 470 K.
Ans: To convert Kelvin (K) to Celsius (°C), we use the following formula:
°C = K – 273.15
(a) 293 K = 293 K – 273.15 ≈ 19.85 °C
(b) 470 X = 470 K – 273.15 ≈ 196.85 °C
Q17. Convert the following temperatures to the Kelvin scale. (a) 25°C (b) 373°C.
Ans: To convert Celsius (°C) to Kelvin (K), we use the following formula:
K = °C + 273.15
(a) 25 °C + 273.15 = 298.15 K
(b) 373 °C + 273.15 = 646.15 K
Q18. Give reason for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several metres away.
Ans: (a) Naphthalene balls disappear with time without leaving any solid because they undergo sublimation.
(b) We can get the smell of perfume sitting several metres away because of diffusion.
Q19. Arrange the following substances in increasing order of forces of attraction between the particles—water, sugar, oxygen.
Ans: The forces of attraction between particles increase in this order:
- Oxygen gas (O₂)
- Water (H₂O)
- Sugar (C₁₂H₂₂O₁₁)
Q20. What is the physical state of water at— (a) 25°C (bj 0°C (cj 100°C
Ans: The physical state of water at the given temperatures are:
(a) 25°C: Liquid (b) 0°C: Solid (ice) (c) 100°C: Mixture of liquid and gas (boiling point)
Q21. Give two reasons to justify
(a) water at room temperature is a liquid.
(b) an iron almirah is a solid at room temperature.
Ans. (a) Water (liquid):
- Strong hydrogen bonding allows movement, but not enough to fix positions completely.
- Sufficient kinetic energy lets molecules overcome attractions to some extent, enabling flow.
(b) Iron almirah (solid):
- Strong metallic bonds hold atoms in a fixed arrangement.
- Low kinetic energy keeps atoms locked in their positions within the solid structure.
Q22. Why is ice at 273 K more effective in cooling than water at the same temperature?
Ans: Ice at 0°C is better for cooling because it absorbs both:
- Sensible heat: Raises temperature (like water).
- Latent heat of fusion: Absorbs heat to change state from ice to water (without increasing temperature), removing more heat overall.
Q23. What produces more severe bums, boiling water or steam?
Ans: Steam burns are worse than boiling water because steam releases extra latent heat when it condenses on your skin, adding to the initial heat and causing more severe burns.
NCERT Solutions for Class 9 Science Chapter 1
FAQ’s
What topics are covered in NCERT Class 9 Science Chapter 1 Class 9 Science Chapter 1?
NCERT Class 9 Science Chapter 1, “Matter in Our Surroundings,” covers the basic concepts of matter, its states (solid, liquid, gas), and physical properties like density, solubility, etc.
What significance does the study of “Matter in Our Surroundings” hold?
Understanding the properties and behavior of matter is fundamental to many scientific disciplines and everyday life. This chapter lays the foundation for further study in chemistry and physics.
How can NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings help me prepare for exams?
NCERT Solutions provide comprehensive explanations, solved examples, and practice questions that can help you understand the concepts thoroughly and prepare effectively for exams.
Are the NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings easy to understand?
Yes, the solutions are designed to be clear and concise, making complex concepts easier to grasp. Additionally, they include step-by-step explanations to aid understanding.
Can I access NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings online?
Yes, NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings are available online, providing convenient access for students to study anytime, anywhere.