1. Preparation (Ostwald Process):
Nitric acid is manufactured commercially by the Ostwald Process. In this method, ammonia is mixed with air and passed over a heated platinum-rhodium gauze catalyst. This oxidizes ammonia to nitric oxide, which is further oxidized and dissolved in water to form nitric acid.
2. Physical Properties:
- It is a fuming, volatile liquid.
- It is colourless in its pure form but often turns yellow due to the dissolution of nitrogen dioxide.
- It has a characteristic pungent odour.
- It is highly corrosive and miscible with water.
3. Chemical Properties:
- Acidic Nature: It turns blue litmus red and reacts with bases and carbonates to form salts.
- As an Oxidizing Agent: Nitric acid is a very strong oxidizing agent. It oxidizes non-metals like carbon and sulphur, and metals to their respective nitrates.
- Reaction with Metals: It does not typically liberate hydrogen gas with metals (except Mg and Mn). Instead, it produces nitrogen oxides (like NO₂ or NO) depending on the concentration of the acid and the reactivity of the metal.
- Aqua Regia: A mixture of concentrated nitric acid and hydrochloric acid in a 1:3 ratio is called Aqua Regia. It can dissolve even noble metals like gold and platinum.
4. Uses:
- Manufacturing of fertilizers (ammonium nitrate).
- Production of explosives (TNT, nitroglycerin).
- In the making of dyes, plastics, and drugs.
- As a laboratory reagent.
EXERCISE .1
1) What is: (a) aqua fortis, (b) aqua regia (c) Fixation of Nitrogen?
Ans: (a) Aqua Fortis
Aqua Fortis is the historical (alchemical) name for concentrated nitric acid (HNO₃). The name translates to “strong water,” and it was known for its powerful ability to dissolve metals like silver and copper.
(b) Aqua Regia
Aqua Regia is a highly corrosive mixture of concentrated nitric acid and concentrated hydrochloric acid, typically in a 1:3 ratio. Its name means “royal water,” referring to its unique ability to dissolve noble metals, such as gold and platinum, which single acids cannot.
(c) Fixation of Nitrogen
Fixation of Nitrogen is the chemical process of converting unreactive atmospheric nitrogen gas (N₂) into useful nitrogen compounds, such as ammonia (NH₃) or nitrates. This process is crucial for life because it makes nitrogen available for plants to use in creating essential proteins and nucleic acids.
2) During thunderstorms, rain water contains nitric acid. Explain with reactions.
Ans: During thunderstorms, the intense energy from lightning causes the main gases in the air—nitrogen (N₂) and oxygen (O₂)—to react.
Step 1: Formation of Nitric Oxide
The high temperature of a lightning bolt forces nitrogen and oxygen to combine, forming nitric oxide (NO).
Reaction: N₂ (g) + O₂ (g) → 2NO (g)
Step 2: Formation of Nitrogen Dioxide
In the air, nitric oxide (NO) quickly reacts with more oxygen to form nitrogen dioxide (NO₂), a brownish gas.
Reaction: 2NO (g) + O₂ (g) → 2NO₂ (g)
Step 3: Formation of Nitric Acid
Nitrogen dioxide (NO₂) then dissolves and reacts with rainwater (H₂O) to form nitric acid (HNO₃), making the rainwater slightly acidic.
Reaction: 3NO₂ (g) + H₂O (l) → 2HNO₃ (aq) + NO (g)
This is why rainwater collected during a thunderstorm is found to contain traces of nitric acid.
3) (a) Write a balanced chemical equation for the laboratory preparation of nitric acid
(b) In the preparation of nitric acid from KNO3 concentrated hydrochloric acid is not used in place of concentrated sulphuric acid. Explain why?
(c) Conc. Nitric acid prepared in the laboratory is yellow in colour. Why? How is this colour removed?
(d) Give reasons for the following: In the laboratory preparation of nitric acid, the mixture of concentrated sulphuric acid and sodium nitrate should not be heated very strongly above 200°C.
Ans: (a) Balanced chemical equation for laboratory preparation of nitric acid:
NaNO₃ + H₂SO₄ (conc.) → NaHSO₄ + HNO₃
(b) Why concentrated HCl is not used instead of concentrated H₂SO₄?
Concentrated hydrochloric acid is not used because:
It is volatile, so it does not displace the less volatile nitric acid effectively.
The reaction would produce nitrosyl chloride (NOCl) and chlorine gas as side products, resulting in an impure acid.
(c) Why conc. HNO₃ is yellow and how is the colour removed?
Reason for yellow colour: On standing or heating, concentrated nitric acid slightly decomposes to form nitrogen dioxide (NO₂), a brown gas. This gas dissolves in the acid, giving it a yellow colour.
Removal of colour: The yellow colour can be removed by bubbling a stream of dry air through the warm acid. The air expels the dissolved nitrogen dioxide gas.
(d) Why should the mixture not be heated strongly above 200°C?
If the mixture of concentrated sulphuric acid and sodium nitrate is heated very strongly (above 200°C), the sodium bisulphate (NaHSO₄) formed can react further with sodium nitrate.
NaNO₃ + NaHSO₄ → Na₂SO₄ + HNO₃
This reaction occurs at a higher temperature. The nitric acid produced at this high temperature decomposes immediately into nitrogen dioxide, resulting in a loss of the product.
4) Nitric acid cannot be concentrated beyond 68% by the distillation of a dilute solution of HNO3 State.
Ans: Nitric acid and water form a special mixture called a constant-boiling azeotrope at approximately 68% HNO₃ and 32% H₂O.
When this 68% solution is heated, both the acid and the water evaporate together in this fixed ratio. The vapor produced has the same concentration (68%) as the liquid.
Therefore, no matter how long you distill a dilute nitric acid solution, the distillate (the condensed vapor) will never be stronger than 68%. The water cannot be fully separated from the acid through simple distillation.
5) What is passive iron? How is passivity removed?
Ans: Passive iron is ordinary iron or steel that has been made highly corrosion-resistant by forming a thin, invisible, and protective oxide layer on its surface. This layer, often made of ferric oxide (Fe₂O₃), acts as a shield that prevents the underlying metal from reacting with water and oxygen in the environment.It is not a special type of iron, but rather a state that iron enters when this protective film is stable and intact.
How is Passivity Removed?
Passivity is removed by destroying this protective oxide layer. This can be done through several methods:
- Mechanical Damage: Scratching, abrading, or grinding the surface physically removes the passive film.
- Chemical Reduction: Using strong reducing agents that can break down the oxide layer.
- Cathodic Protection: Applying an external electrical current that suppresses the formation of the passive layer.
- Introduction of Aggressive Ions: The most common method involves exposing the passive iron to specific ions, especially chloride ions (found in seawater and salt). These ions penetrate the oxide film, break it down locally, and cause pitting corrosion, thus removing the passive state.
6) Name the products formed when:
(a) carbon and conc. Nitric acid is heated
(b) dilute HNO3 is added to copper.
Ans: (a) C + 4HNO3—> CO2 + 2H2O +4NO2
(b) 3Cu + 8HNO3—> 3Cu(NO3) 2 + 4H2O + 2NO
7) Give two chemical equations for each of the following:
(a) Reactions of nitric acid with non-metals
(b) Nitric acid showing as acidic character
(c) Nitric acid acting as oxidizing agent
Ans: (a) Reaction of nitric acid with non-metals:
C + 4HNO3→ CO2 + 2H2O + 4 NO2
S + 6 HNO3 → H2SO4 + 2H2O + 6 NO2
(b) Nitric acid showing acidic character:
K2O + 2HNO3→ 2KNO3 + H2O
ZnO + 2HNO3 → Zn(NO3)2 + H2O
(c) Nitric acid acting as oxidizing agent
P4 +20HNO3 → 4H3PO4 + 4H2O + 20NO2
3Zn +8HNO3→ 3Zn(NO3)2 + 4H2O + 2NO
8) Write balanced equations and name the products formed when:
(a) Sodium hydrogen carbonate is added to nitric acid
(b) cupric oxide reacts with nitric acid
(c) zinc reacts with dilute nitric acid
(d) concentrated nitric acid is heated
Ans: (a) When sodium hydrogen carbonate is added to nitric acid sodium nitrate, carbon dioxide and water is formed.
NaHCO3 + HNO3 —> NaNO3 + H2O + CO2
(b) When Cupric oxide reacts with dilute nitric acid, it forms Copper nitrate.
CuO + 2HNO3—> Cu(NO3)2 + H2O
(c) Zinc reacts with nitric acid to form Zinc nitrate, nitric oxide and water.
3 Zn + 8HNO3 —> 3Zn(NO3)2 + 4H2O + 2NO
(d) 4HNO3—> 2H2O + 4NO2 + O
9) Write an equation for the following conversions A, B, C and D.
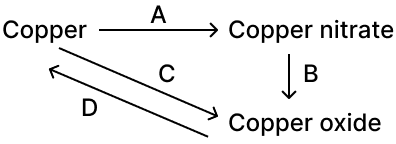
Ans:
A: Copper can be converted into copper nitrate.
3Cu + 8HNO3—> 3Cu(NO3)2 + 4H2O + 2NO
B: 2Cu(NO3)2 —> 2CuO + 4NO2 + O2
C: 2Cu + O2—> 2CuO
D: By reduction 2CuO + C—> 2Cu + CO2
10) How will you prepare the following from nitric acid?
(a) Sodium nitrate
(b) copper nitrate
(c) Lead nitrate
(d) Magnesium nitrate
(e) Ferric nitrate
(f) Aqua regia
Ans: (a) Sodium nitrate: NaOH + HNO3 —> NaNO3 + H2O Sodium hydroxide reacts with nitric acid to form sodium nitrate.
(b) Copper nitrate: CuO + 2HNO3 —> Cu(NO3)2 + H2O Copper oxide reacts with nitric acid to form copper nitrate.
(c) Lead nitrate: Pb + 4HNO3—> Pb(NO3)2 + 2H2O + 2NO2 Lead reacts with conc. nitric acid to form lead nitrate.
(d) Magnesium nitrate: Mg + 2HNO3—> Mg(NO3)2 + H2 Magnesium with dil. nitric acid to form magnesium nitrate.
(e) Ferric nitrate: Fe + 6HNO3—> Fe(NO3)3 + 3H2O + 3NO2
Iron reacts with conc. nitric acid to form ferric nitrate.
(f) Aqua regia: HNO3 + 3HCl —> NOCl + 2H2O + 2[Cl] Nitric acid reacts with hydrochloric acid to form a mixture called aqua regia.
11) Correct the following, if required:
(a) HNO3 is a strong reducing agent.
(b) NaNO3 gives Na2 and O2 on heating.
(c) Constant boiling nitric acid contains 80% nitric acid by weight.
(d) Nitric acid remains colourless even when exposed to light.
Ans: (a) Incorrect. HNO₃ is a strong oxidizing agent, not a reducing agent.
(b) Incorrect. NaNO₃ on heating gives NaNO₂ and O₂, not Na₂ and O₂.
(c) Incorrect. Constant boiling nitric acid contains about 68% nitric acid by weight.
(d) Incorrect. Nitric acid turns yellow on exposure to light due to decomposition into NO₂.
12) Name: (a) a nitrate of metal which on heating does not give nitrogen dioxide
(b) a nitrate which on heating leaves no residue behind.
(c) a metal nitrate which on heating is changed into metal oxide
(d) a metal nitrate which on heating is changed into metal
(e) a solution which absorbs nitric oxide
(f) the oxide of nitrogen which turns brown on exposure to air. How is it prepared?
Ans: (a) Sodium nitrate
On heating, sodium nitrate decomposes to sodium nitrite and oxygen, without producing nitrogen dioxide.
(b) Ammonium nitrate
On heating, it decomposes into nitrous oxide and water vapor, leaving no solid residue.
(c) Copper nitrate
On heating, copper nitrate decomposes to give copper oxide, nitrogen dioxide, and oxygen.
(d) Silver nitrate
On heating, silver nitrate decomposes to silver metal, nitrogen dioxide, and oxygen.
(e) Ferrous sulphate solution
It absorbs nitric oxide (NO) to form a dark brown complex, FeSO₄·NO.
(f) Nitrogen monoxide (NO)
It turns brown on exposure to air due to the formation of nitrogen dioxide (NO₂).
Preparation:
It is prepared by the action of dilute nitric acid on copper:
3Cu+8HNO3(dilute)→3Cu(NO3)2+2NO+4H2O
13) Give the chemical name and formula of the substance formed as a brown ring in the test for nitrate radical.
Ans: The brown ring formed in the nitrate test is a complex compound named Nitrosyl Ferrous Sulphate.
Its chemical formula is [Fe(NO)(H₂O)₅]SO₄.
It is formed when ferrous sulphate reacts with nitric oxide generated during the test.
14)Mention three important uses of nitric acid. Give the property of nitric acid involved in the use.
Ans: Manufacture of Fertilizers (e.g., Ammonium Nitrate):
Use: To produce nitrogen-based fertilizers.
Property: It is a strong acid that reacts with ammonia (a base) in a neutralization reaction to form ammonium nitrate salt.
Manufacture of Explosives (e.g., TNT, Nitroglycerin):
Use: To introduce nitro groups (-NO₂) into organic compounds.
Property: It is a strong oxidizing agent and participates in nitration reactions, making organic compounds highly unstable and explosive.
Purification of Metals (e.g., Gold, Silver):
Use: To separate gold from other metals like silver in aqua regia.
Property: It is a strong oxidizing agent. In aqua regia (a mixture with HCl), it oxidizes gold to form soluble ions, allowing it to be dissolved and separated.
15) Choose the correct answer:
(a) The nitrate salt which does not give a mixture of NO2 and O2 on heating is:
(i) AgNO3
(ii) KNO3
(iii) Cu(NO3)2
(iv) Zn(NO3)2
(b) The chemical used in the brown ring test is:
(i) CuSO4 (ii) FeSO4 (iii) Fe2(SO4)3 (iv) N2O
Ans: (a) KNO3
(b) FeSO4
(c) NO2
16) (a) Explain with the help of a balanced equation, the brown ring test for nitric acid.
(b) Why is freshly prepared ferrous sulphate solution used for testing the nitrate radical in the brown ring test?
Ans: (a) Brown Ring Test for Nitric Acid
This ring is made of a compound with the formula FeSO₄·NO, known as the brown ring compound. It forms when concentrated sulphuric acid reacts with the nitrate ions to produce nitrogen dioxide. This nitrogen dioxide then reacts with the ferrous ions (Fe²⁺) from the FeSO₄ solution to create the characteristic brown-colored complex at the junction of the liquids.
The balanced chemical equation for the reaction is:
6FeSO₄ + 3H₂SO₄ + 2HNO₃ → 2Fe₂(SO₄)₃ + 4H₂O + 2NO
The nitric oxide (NO) gas produced then reacts with ferrous sulphate to form a brown-colored complex compound called FeSO₄·NO (or [Fe(H₂O)₅NO]SO₄), which is the “brown ring”.
(b) Reason for using Freshly Prepared Ferrous Sulphate
Freshly prepared ferrous sulphate solution is used because ferrous ions (Fe²⁺) are easily oxidized by atmospheric oxygen to ferric ions (Fe³⁺) on standing.
If an old or oxidized solution is used, most of the Fe²⁺ ions would have already turned into Fe³⁺ ions. Since the brown ring complex forms only with Fe²⁺ ions, an old solution would give a false negative result or a very faint ring, failing to detect the presence of nitrate ions.
17) From the following list of substances, choose one substance in each case which matches the description given below: Ammonium nitrate, calcium hydrogen carbonate, copper carbonate, lead nitrate, potassium nitrate, sodium carbonate, sodium hydrogen carbonate, zinc carbonate.
(a) A nitrate which gives off only oxygen when heated
(b) A nitrate which on heating decomposes into dinitrogen oxide [nitrous oxide] and steam.
(c) A nitrate which gives off oxygen and nitrogen dioxide when heated.
Ans: (a) Potassium nitrate
(b) Ammonium nitrate
(c) Lead nitrate
18) Fill in the blanks: (a) Aqua regia is a mixture of 3 parts ———— and one part ————.
(b) The catalytic oxidation of ammonia to nitric oxide is ————. (exothermic / endothermic) process.
(c) Magnesium gives ————(O2, H2, NO) with very dilute nitric acid.
(d) ———— (iron / copper) become passive in concentrated nitric acid
Ans: (a) Aqua regia is a mixture of 3 parts Hydrochloric acid and one part Nitric acid.
(b) The catalytic oxidation of ammonia to nitric oxide is exothermic.
(c) Magnesium gives H2 with very dilute nitric acid.
(d) Iron become passive in concentrated nitric acid
19) The action of heat on the blue crystalline solid A, gives a reddish brown gas B, a gas which relight a glowing splint and leaves a black residue. When gas C, which has a rotten egg smell, is passed through a solution of A, a black ppt. is formed.
(a) Identify A, B and C
(b) Write an equation for action of heat on A.
(c) Write an equation between the solution of A and gas C.
Ans: (a) Identification
A is Copper(II) Nitrate, Cu(NO₃)₂·6H₂O (a blue crystalline solid).
B is Nitrogen Dioxide, NO₂ (a reddish-brown gas).
C is Hydrogen Sulphide, H₂S (a gas with a rotten egg smell).
(b) Action of Heat on A
When Copper(II) Nitrate is heated strongly, it decomposes.
The balanced chemical equation is:
2Cu(NO3)2(s)→Δ4NO2(g)+O2(g)+2CuO(s
The reddish-brown gas (B) produced is Nitrogen Dioxide (NO₂).Oxygen (O₂) gas is also released, which will re-light a glowing splint.A black residue of Copper(II) Oxide (CuO) is left behind.
(c) Reaction between Solution of A and Gas C
When a solution of Copper(II) Nitrate reacts with Hydrogen Sulphide gas, a double displacement reaction occurs, forming a black precipitate.
The balanced chemical equation is:
Cu(NO3)2(aq)+H2S(g)→CuS(s)+HNO3(aq)
The black precipitate is Copper(II) Sulphide (CuS).
Question 1(2004): X, Y and Z are three crystalline solids which are soluble in water and have a common anion. To help you to identify X, Y and Z you are provided with the following experimental observations. Copy and complete the corresponding inferences in (a) to (f). (a) A reddish-brown gas is obtained when X, Y and Z are separately warmed with concentrated sulphuric acid and copper turning added to the mixture.
INFERENCE 1 : The common anion is the …………… ion. (b) When X is heated, it melts and gives off only one gas which re-lights a glowing splint.
INFERENCE 2: The cation in X is either ……………. Or …………….. (c) The action of heat on Y produces a reddish-brown gas and a yellow residue which fuses with the glass of the test tube.
INFERENCE 3: The metal ion present in Y is the …………….. ion (d) When Z is heated, it leaves no residue. Warming Z with sodium hydroxide solution liberates a gas which turns moist red litmus paper blue.
INFERENCE 4: Z contains the ……………. Cation. (e) Write the equations for the following reactions:
(1) X and concentrated sulphuric acid (below 200° C). (One equation only for either of the cations given in INFERENCE 2). (2) Action of heat on Y. (3) Concentrated nitric acid is added to copper turnings kept in a beaker.
(a) Nitrate. (b) Sodium or potassium (c) Lead (d) Ammonia (e) (1) KNO3 + H2SO4 —>KHSO4 + HNO3 (f) 2Pb(NO3)2—> 2PbO + 4NO2 +O2 (g) Cu +4HNO3—> Cu(NO3)2 +H2O +2NO2
Ans: (a) INFERENCE 1: The common anion is the nitrate ion.
(b) INFERENCE 2: The cation in X is either sodium or potassium.
(c) INFERENCE 3: The metal ion present in Y is the lead ion.
(d) INFERENCE 4: Z contains the ammonium cation.
(e) (1) KNO3 + H2SO4 —> KHSO4 + HNO3
(f) 2Pb(NO3)2—> 2PbO + 4NO2 +O2
(g) Cu +4HNO3 —> Cu(NO3)2 +H2O +2NO2
Question 1(2005):
(a) Dilute acid is generally considered a typical acid except for its reaction with metals. In what way is dilute nitric acid different from other acids when it reacts with metals?
(b) Write the equation for the reaction of dilute nitric acid with copper.
Ans: (a)
Dilute nitric acid acts as a strong oxidizing agent. Unlike most dilute acids, it does not release hydrogen gas when reacting with metals. Instead, it gets reduced to nitrogen oxides such as nitric oxide (NO).
(b)
Copper reacts with dilute nitric acid as follows:
3Cu + 8HNO₃(dilute) → 3Cu(NO₃)₂ + 2NO + 4H₂O
Question 1(2006): Explain why:
(a) Only all – glass apparatus should be used for the preparation of nitric acid by heating concentrated sulphuric acid and potassium nitrate.
(b) Nitric acid is kept in a reagent bottle for a long time.
Ans: (a) Why only all-glass apparatus should be used:
Hot, concentrated nitric acid produced in this reaction is a powerful oxidizing agent. It attacks and damages non-glass materials like rubber, plastic, or cork, leading to contamination and potential breakage. Glass is inert and withstands the high temperatures and corrosive nature of the acid.
(b) Why nitric acid turns yellow upon long storage:
Nitric acid slowly decomposes over time, especially when exposed to light, releasing nitrogen dioxide (NO₂) gas. This brown-colored gas dissolves in the acid, giving it a yellow or brownish tint. The color deepens as more gas is produced.
Question 2(2006): Write a chemical equation to illustrate the acidic nature of nitric acid.
Ans: The chemical equation:
HNO3 H+ + NO3−
Question 3(2006): Name the products formed when ammonium nitrate is heated.
Ans: When ammonium nitrate is heated, it decomposes to form nitrous oxide (also known as dinitrogen monoxide) and water vapor.
The chemical reaction is:
Ammonium Nitrate → Nitrous Oxide + Water
Question 1(2007): The figure given below illustrates the apparatus used in the laboratory preparation of nitric acid.
(a) Name A (a liquid), B (a solid) and C (a liquid). ( Do not give the formulae) (b) Write an equation to show how nitric acid undergoes decomposition (c) Write the equation for the reaction in which copper is oxidized by concentrated nitric acid
Ans: (a) Names:
A (Liquid): Concentrated Nitric Acid
B (Solid): Potassium Nitrate
C (Liquid): Concentrated Sulfuric Acid
(b) Decomposition of Nitric Acid:
Nitric acid breaks down into nitrogen dioxide, water, and oxygen.
4HNO3→4NO2+2H2O+O2
(c) Oxidation of Copper:
Copper reacts with concentrated nitric acid, producing a brown gas.
Cu+4HNO3→Cu(NO3)2+2NO2+2H2O
Question 1(2008): (a) A dilute acid B does not normally give hydrogen when reacted with metals but does give a gas when reacts with copper. Identify B. Write equation with copper.
(b) Complete the table:
| Name of process | Inputs | Equation | Output |
| Ammonia + Air | Nitric acid |
Ans: (a) B is Nitric acid (HNO₃).
It acts as an oxidizing agent, so it generally does not produce hydrogen gas with metals.
With copper, it gives nitrogen monoxide gas.
Reaction with copper:
3Cu+8HNO3(dil.)→3Cu(NO3)2+2NO+4H2O
(b)
| Name of process | Inputs | Equation | Output |
| Ostwald process | Ammonia + Air | 4NH 5O 4NO 6HO Heat 0 50C 2 2 2NO O 2NO 2 2 2 3 4NO 2HO O 4HNO | Nitric acid |
(c) Copper doesn’t react with most acids like hydrochloric acid (HCl) because it isn’t reactive enough. For a typical acid-metal reaction to work, the metal must be able to push electrons onto the H⁺ ions to form hydrogen gas. Copper, being a less reactive metal, cannot do this.
Nitric acid (HNO₃) is different because it is a powerful oxidizing agent. Its strength doesn’t come from its H⁺ ions, but from the nitrate ion (NO₃⁻). The nitrogen in this ion is highly electron-hungry.
When copper is added, the nitrate ion aggressively pulls electrons away from the copper metal. This oxidizes the copper, turning it into blue copper(II) ions. Simultaneously, the nitrate ion itself gets reduced, forming brown fumes of nitrogen dioxide gas.
In essence, nitric acid bypasses the need for a simple hydrogen-producing reaction. It attacks copper directly through a powerful redox (oxidation-reduction) process, which is why the vigorous reaction occurs.