1. Conduction
This is the process where heat travels through a substance without the movement of the material itself.
It occurs mainly in solids.
How it works: When one part of a solid is heated, its particles vibrate faster. These vibrations are passed on to neighbouring particles, transferring heat along the object.
Example: A metal spoon becoming hot when one end is kept in a hot tea.
Conductors: Materials like metals that allow heat to pass through them easily.
Insulators: Materials like wood, plastic, and air that do not allow heat to pass through easily.
2. Convection
This is the process where heat is transferred by the actual movement of the heated material (liquid or gas).
It occurs in liquids and gases.
How it works: When a fluid is heated, it expands, becomes lighter, and rises. The colder, denser fluid moves down to take its place.
Example: Boiling water in a pot, sea breezes and land breezes.
3. Radiation
This is the process where heat is transferred without any medium. It does not require particles to carry the heat.
It can travel through a vacuum.
How it works: Heat travels in the form of invisible waves called infrared radiation.
Example: We feel the heat from the Sun, which travels through the vacuum of space.
Key Applications:
Sea Breeze: During the day, the land heats up faster than water. The hot air over the land rises, and cooler air from the sea rushes in, creating a sea breeze.
Land Breeze: At night, the land cools faster than water. The warmer air over the sea rises, and cooler air from the land moves towards the sea, creating a land breeze.
Thermos Flask: It minimizes heat transfer by using a vacuum (to stop conduction and convection) and silvered walls (to reflect radiant heat back).
Test your self
A. Objective Questions
1. Write true or false for each statement
(a) Evaporation is rapid on a wet day.
Ans: False.
(b) Evaporation takes place only from the surface of liquid.
Ans:True.
(c) All molecules of a liquid take part in the process of evaporation.
Ans: False.
(d) Temperature of a liquid rises during boiling or vaporization
Ans: False.
(e) All molecules of a liquid take part in boiling.
Ans: True.
(f) Boiling is a rapid phenomenon.
Ans: True.
(g) All solids expand by the same amount when heated to the same rise in temperature.
Ans: False.
(h) Telephone wires are kept tight between the two poles in winter.
Ans:True.
(i) Equal volumes of different liquids expand by the different amount when they are heated to the same rise in temperature.
Ans: True.
(j) Solids expand the least and gases expand the most on being heated.
Ans: True.
(k) A mercury thermometer makes use of the property of expansion of liquids on heating.
Ans: True.
(l) Kerosene contracts on heating.
Ans:. False.
2. Fill in the blanks
(a) Boiling occurs at a fixed temperature.
(b) Evaporation takes place at all temperatures.
(c) The molecules of liquid absorb heat from surroundings in evaporation.
(d) Heat is absorbed during boiling.
(e) Cooling is produced in evaporation.
(f) A longer rod expands more than a shorter rod on being heated to the same temperature.
(g) Liquids expand more than the solids.
(h) Gases expand more than liquids.
(i) Alcohol expands more than water.
(j) Iron expands less than copper.
Ans:
(a) Boiling occurs at a fixed temperature.
(b) Evaporation takes place at all temperatures.
(c) The molecules of liquid absorb heat from surroundings in evaporation.
(d) Heat is absorbed during boiling.
(e) Cooling is produced in evaporation.
(f) A longer rod expands more than a shorter rod on being heated to the same temperature.
(g) Liquids expand more than the solids.
(h) Gases expand more than liquids.
(i) Alcohol expands more than water.
(j) Iron expands less than copper.
3. Match the following


4. Select the correct alternative
(a) In evaporation
- all molecules of liquid begin to escape out
- only the molecules at the surface escape out
- the temperature of liquid rises by absorbing heat from surroundings.
- the molecules get attracted within the liquid.
Ans: only the molecules at the surface escape out
(b) The rate of evaporation of a liquid increases when :
- temperature of liquid falls
- liquid is poured in a vessel of less surface area
- air is blown above the surface of liquid
- humidity increases.
Ans: air is blown above the surface of liquid
(c) During boiling or vaporization
- all molecules take part
- temperature rises
- no heat is absorbed
- the average kinetic energy of molecules increases.
Ans: all molecules take part
(d) The boiling point of a liquid is increased by
- increasing the volume of liquid
- increasing the pressure, on liquid
- adding ice to the liquid
- decreasing pressure on liquid.
Ans: increasing the pressure, on liquid
(e) Two rods A and B of the same metal, but of length 1 m and 2 m respectively, are heated from 0°C to 100°C. Then
- both the rods A and B elongate the same
- the rod A elongates more than the rod B
- the rod B elongates more than the rod A
- the rod A elongates, but the rod B contracts.
Ans: the rod B elongates more than the rod A
(f) Two rods A and B of the same metal, same length, but one solid and the other hollow, are heated to the same rise in temperature.
Then
- the solid rod A expands more than the hollow rod B
- the hollow rod B expands more than the solid rod A
- the hollow rod B contracts, but the solid rod A expands
- both the rods A and B expand the same.
Ans: both the rods A and B expand the same.
(g) A given volume of alcohol and the same volume of water are heated from the room temperature to the same temperature then.
- alcohol contracts, but water expands
- water contracts, but alcohol expands
- water expands more than alcohol
- alcohol expands more than water.
Ans: alcohol expands more than water.
(h) The increase in length of a metal rod depends on
- the initial length of the rod only
- the rise in temperature only
- the material of rod only
- all the above three factors.
Ans: all the above three factors.
(i) The correct statement is
- Iron rims are cooled before they are placed on the cart wheels.
- A glass stopper gets tightened on warming the neck of the bottle.
- Telephone wires sag in winter, but become tight in summer.
- A little space is left between two rails on a railway track.
Ans: A little space is left between two rails on a railway track.
B. Short/Long Answer Questions
Question 1. What does matter ? What is it composed of
Ans: Matter is simply the stuff that everything is made of. For something to be considered matter, it must have two key properties: it must have some mass (meaning it can be weighed), and it must take up space (meaning it has volume).
Look around you. The pen in your hand, the water you drink, and even the air you breathe—all of these are different forms of matter.
All matter, regardless of what it is, is built from incredibly small particles called atoms. Think of atoms as the fundamental building blocks of everything. These atoms can join together in various ways to form molecules, which give different materials their unique properties. For example, two hydrogen atoms and one oxygen atom join to form a water molecule.
Going even deeper, atoms themselves are made up of a central nucleus (containing protons and neutrons) surrounded by electrons whizzing around it. It is the arrangement and type of these tiny particles that ultimately determine what kind of matter we have.
Question 2. Name the three states of matter and distinguish them on the basis of their (i) volume, and (ii) shape
Ans:The three common states of matter are solid, liquid, and gas. We can tell them apart by looking at two simple properties: their shape and their volume.
1. Solids
A solid has a fixed shape that does not change on its own. It also has a fixed volume. For example, a stone remains a stone, keeping its shape and size whether it is on a table or in your hand.
2. Liquids
A liquid does not have a fixed shape. It takes the shape of its container. If you pour water from a glass into a flat bowl, it will spread out to form a puddle. However, a liquid does have a fixed volume. The same amount of water will fill the bottom of the bowl; it does not expand to fill the entire space.
3. Gases
It will completely fill any closed container it is placed in. For instance, the air in a balloon takes the balloon’s shape and will expand to fill a bigger balloon if transferred.
Question 3. Distinguish between liquid and vapour (or gas) states of matter on the basis of following factors (a) Arrangement of molecules (b) Inter-molecular separation (c) Inter-molecular force, and (d) Kinetic energy of molecules
Ans:
| Factors | liquid state | Vapour State |
| (a) Arrangement of molecules | Molecules are less orderly and can slide past each other. | Molecules are in a completely random and disordered arrangement. |
| (b) Inter-molecular separation | Molecules are quite close together. | Molecules are very far apart compared to their size. |
| (c) Inter-molecular force | Strong inter-molecular forces hold the molecules together. | Inter-molecular forces are extremely weak, almost negligible. |
| (d) Kinetic energy of molecules | Molecules have moderate kinetic energy. | Molecules have very high kinetic energy and move very rapidly |
Question 4. What is evaporation ? Explain it on the basis of molecular motion.
Ans: Evaporation is the process where a liquid turns into vapor from its surface, without boiling.
Molecular Explanation:
Inside a liquid, molecules move constantly with different energies.The molecules with the highest energy near the surface can overcome the attractive forces of other molecules.These high-energy molecules break free and escape as vapour.The molecules left behind now have a lower average energy.Since temperature is related to this average energy, the liquid cools down.This is why evaporation causes a cooling effect, like when sweat dries from your skin.
Question 5. Do all the molecules of a liquid take part in evaporation ? If not, explain your answer.
Ans: No, not all the molecules of a liquid take part in evaporation at the same time.
Evaporation is a surface phenomenon. It occurs when molecules at the surface of the liquid gain enough kinetic energy to overcome the intermolecular forces of attraction and escape into the air as vapour.Molecules within the bulk of the liquid do not evaporate directly because they are surrounded by other molecules on all sides. The strong intermolecular forces from these surrounding molecules trap them inside the liquid. Only the molecules on the surface, which have fewer neighbouring molecules holding them back, have a chance to escape.
Question 6. No heat is supplied to a liquid during evaporation. How does then the liquid change into its vapours ?
Ans: During evaporation, the liquid changes into vapour without an external heat supply because the process uses internal energy.The more energetic (hotter) molecules in the liquid possess enough kinetic energy to break the surface tension and escape as vapour. When these high-energy molecules leave, the average energy of the remaining liquid decreases, which is why evaporation causes cooling. The latent heat of vaporization is drawn from the liquid itself.
Question 7. Comment on the statement ‘evaporation is a surface phenomenon’.

Ans: Evaporation is a surface phenomenon. It occurs when molecules at the surface of a liquid gain enough energy to break away and turn into vapour. Molecules inside the liquid cannot escape directly into the air; only the molecules on the surface can undergo this process.
Question 8. Why is cooling produced when a liquid evaporates ?
Ans: When a liquid evaporates, cooling is produced because the process requires energy.The more energetic molecules in the liquid escape as vapour, taking their heat energy with them. This leaves the remaining liquid with less energetic molecules, which means it has a lower average kinetic energy and hence, a lower temperature.In simple terms, the liquid uses heat from its own surroundings to turn into vapour, making the surroundings cooler.
Question 9. Give reason for the increase in rate of evaporation of a liquid when (a) air is blown above the liquid (b) surface area of liquid is increased (c) temperature of liquid is increased.
Ans: (a) When air is blown above the liquid:
Blowing air removes the water vapour molecules present just above the liquid’s surface. This reduces the humidity in the surrounding air, making it easier for more liquid molecules to escape into the air, thus increasing evaporation.
(b) When the surface area of the liquid is increased:
Evaporation is a surface phenomenon. A larger surface area (like spreading a cloth to dry) exposes more liquid molecules to the air at the surface. This gives a greater number of molecules the opportunity to escape, speeding up evaporation.
(c) When the temperature of the liquid is increased:
On heating, the average kinetic energy of the liquid molecules increases. More molecules gain enough energy to overcome the forces of attraction from other molecules and break away from the liquid surface into the air, leading to faster evaporation.
Question 10. What is boiling ? Explain it on the basis of molecular motion?
Ans: When a liquid reaches its boiling point, the heat energy being supplied does not raise the temperature further. Instead, this energy makes the molecules within the liquid move much more violently.This intense motion gives each molecule enough energy to overcome the attractive forces that were holding it to its neighboring molecules in the liquid state. As a result, pockets of the liquid turn into a gas, forming bubbles of vapour right inside the liquid, not just at the surfaceThese bubbles, being less dense than the liquid, then rush upwards to the surface. When they break through the surface, the gas inside escapes into the air. It is this rapid formation and rise of countless vapour bubbles that we see as the vigorous bubbling during boiling.
Question 11. Why does bubbles appear when a liquid is heated ?
Ans: Bubbles appear when a liquid is heated for two main reasons:
Before Boiling: The tiny bubbles are dissolved air (like oxygen and nitrogen) escaping from the liquid. This happens because hot water cannot hold as much dissolved gas as cold water.
During Boiling: The large, rolling bubbles are the liquid itself turning into vapor or steam. These vapor bubbles form at the boiling point, rise to the surface, and burst.
Question 12. What is the change in average kinetic energy of molecules of a liquid during boiling at its boiling point ?
Ans: During boiling, the temperature of a liquid remains constant, even though heat is continuously supplied. This occurs because the heat energy is not used to increase the kinetic energy or speed of the molecules, which would raise the temperature. Instead, this energy is used to overcome the intermolecular forces of attraction between the liquid particles. This process enables the molecules to break free from the liquid state and transform into vapour. Since the supplied heat is utilized solely for this change of state (from liquid to gas) and not for increasing molecular motion, the temperature does not rise and stays constant at the boiling point.
Question 13. How is the heat energy supplied to a liquid used during boiling at a fixed temperature ?
Ans: The statement accurately describes what happens during boiling.When we heat a liquid, its temperature rises until it reaches its boiling point. At this stage, a crucial change occurs. The heat energy we continue to supply no longer increases the temperature reading on the thermometer Instead, this incoming energy is entirely used for a different purpose: to break the strong forces of attraction (intermolecular forces) that hold the liquid particles together. As these bonds are broken, the particles are able to move apart freely and change their state from a liquid to a gas (vapour).This “hidden” or “stored” energy, which is used purely for changing the state without causing a temperature change, is precisely what we call the latent heat of vaporization.
Question 14. Name two ways of change of liquid state to the vapour state and distinguish them.
Ans: The two ways of change from liquid state to vapour state are evaporation and boiling.
Evaporation is a slow, surface phenomenon that occurs at any temperature below the boiling point of the liquid. For example, water in a wet cloth dries up at room temperature. Boiling, on the other hand, is a fast, bulk phenomenon that occurs only at a fixed temperature, known as the boiling point. During boiling, vapour bubbles form throughout the liquid, such as when water is heated to 100°C. The main distinction is that evaporation happens at all temperatures from the surface, while boiling happens rapidly at a fixed temperature from the entire liquid mass.
Question 15. What do you understand by thermal expansion of a substance ?
Ans: Thermal expansion is a physical property of a substance. It refers to the increase in the size (length, area, or volume) of a substance when its temperature is increased.When a substance is heated, its particles (atoms or molecules) gain kinetic energy and start vibrating more vigorously. These increased vibrations cause the particles to move slightly farther apart from each other. This average increase in the separation between particles results in the expansion of the entire substance.
Thermal expansion can be observed in all three states of matter:
Solids: Expansion is most noticeable, e.g., railway tracks have small gaps to allow for expansion in summer.
Liquids: Expansion is more than in solids, e.g., mercury in a thermometer rises when heated.
Gases: Expansion is the greatest, e.g., a balloon inflates more when kept in the sun.
Conversely, when the substance is cooled, its particles lose energy and move closer, causing it to contract.
Question 16. Give two examples of the substances which expand on heating.
Ans: Most common substances expand when they are heated. Here are two everyday examples:
Metals: Metals are a common example of expansion on heating. For instance, iron or steel used in railway tracks expands during the hot summer. This is why small gaps are left between two consecutive rails to provide space for this expansion, preventing the tracks from bending.
Liquids (like Mercury or Alcohol): Liquids also expand when heated. This property is used in thermometers. The mercury or coloured alcohol inside the thermometer’s bulb expands and rises up the narrow capillary tube when the temperature increases, allowing us to measure the temperature.
Question 17. Describe an experiment to demonstrate the thermal expansion in solids.
Ans:
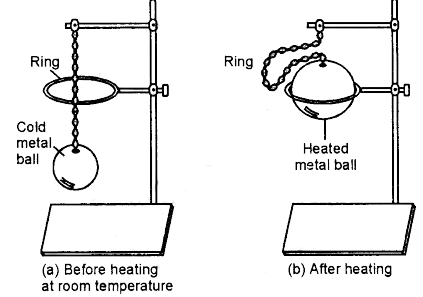
Aim: To demonstrate the thermal expansion in a solid.
Apparatus Required: A metal rod (e.g., iron or aluminium), a clamp stand, a needle, a burner, and a wooden block.
Procedure:
Set up the metal rod horizontally by clamping one of its ends firmly to a stand.
Place a wooden block on the table such that the other, free end of the rod rests on it.
Position the needle vertically between the free end of the rod and the wooden block, so that the rod just touches the tip of the needle. The needle should be able to roll easily.
Mark the initial position of the needle on the table.
Now, heat the metal rod strongly along its length using a burner.
As the rod is heated, observe the movement of the needle.
Observation: After some time, you will see that the needle starts to roll away from its original position.
Conclusion: The heating causes the metal rod to expand in length. Since one end is fixed, the expanding rod pushes the needle forward. This movement clearly shows that solids expand on heating, demonstrating the phenomenon of thermal expansion.
Question 18. State three factors on which depend the linear expansion of a metal rod on heating.
Ans: The linear expansion of a metal rod upon heating depends on the following three factors:
Original Length of the Rod: The increase in length is directly proportional to the original length of the rod. A longer rod will expand more than a shorter rod of the same material when heated through the same temperature range.
Rise in Temperature: The increase in length is directly proportional to the change in temperature. A greater increase in temperature causes a greater increase in the length of the rod.
Material of the Rod (Coefficient of Linear Expansion): Different metals expand by different amounts even for the same original length and same temperature rise. This inherent property is measured by the ‘coefficient of linear expansion’ of the material. For example, aluminium expands more than iron for the same conditions.
Question 19. Two iron rods – one 10 m long and the other 5 m long, are heated to the same rise in temperature. Which will expand more ?
Ans: The 10-meter long iron rod will expand more.
Reason:
The amount of linear expansion in a rod depends directly on its original length. Since both rods are made of the same material (iron) and experience the same rise in temperature, the longer rod (10 m) will undergo a greater increase in length compared to the shorter rod (5 m).
Question 20. Two identical rods of copper are heated to different temperatures — one by 5°C and the other by 10°C. Which rod will expand more ?
Ans: The rod heated by 10°C will expand more.
Reason:
Both rods are identical (same material, same initial length). The expansion of a solid depends directly on the rise in its temperature. Since the second rod experiences twice the temperature increase (10°C vs 5°C), it will undergo approximately twice the linear expansion compared to the first rod.
Question 21. One rod of copper and another identical rod of iron are heated to the same rise in temperature. Which rod will expand more? Give reason.
Ans: The amount by which a material expands on heating depends on its ‘coefficient of linear expansion’. Copper has a higher value for this coefficient compared to iron. This means that for the same increase in temperature and for two identical rods, copper will always expand more in length than iron.
Question 22. Two identical rods—one hollow and the other solid, are heated to the same rise in temperature. Which will expand more ?
Ans: The given statement is incorrect.
Both the hollow and solid rods, being made of the same material and initially having the same length, will expand by the exact same amount when heated through the same temperature rise.
Correct Explanation:
The thermal expansion of a rod (its change in length) depends on three factors:
Its original length.
The temperature change.
The coefficient of linear expansion of the material.
Since both rods are identical in original length, material, and experience the same temperature change, their linear expansion will be identical. The fact that one is hollow and has a larger surface area is irrelevant for linear expansion. The hollow rod will expand in length just as much as the solid one.
Question 23. In the ball and ring experiment, if the ball after heating is left to cool on the ring for some time, the ball again passes through the ring. Explain the reason.
Ans: In the ball and ring experiment, when the heated ball is left to cool on the ring, both objects are in direct contact. The hot ball transfers its heat to the cooler metal ring. As the ring absorbs this heat, it undergoes thermal expansion, causing its inner diameter to increase.
Simultaneously, the ball loses heat to the ring and the surrounding air, causing it to contract.Therefore, after some time, two changes happen together:
The ball shrinks (contracts) back to a smaller size.
The ring expands to a larger inner diameter.
Due to this combined effect of the ball contracting and the ring expanding, the ball can once again pass through the ring. This demonstrates the principle of thermal contraction.
Question 24. Explain the following: (a) The telephone wires break in winter. (b) Iron rims are heated before they are fixed on the wooden wheels. (c) The gaps are left between the successive rails on a railway track. (d) A glass stopper stuck in the neck of a bottle can be removed by pouring hot water on the neck of the bottle. (e) A cement floor is laid in small pieces with gaps in between.
Ans: (a) The telephone wires break in winter.
Telephone wires are made of metal, which contracts (becomes shorter) in cold weather. In winter, the temperature drops significantly, causing the wires to contract with a great deal of force. If they are too tight to begin with, this contraction can create excessive tension, ultimately leading to the wires snapping or breaking.
(b) Iron rims are heated before they are fixed on the wooden wheels.
This is a common technique using the principle of expansion. The iron rim is heated, causing it to expand in size. In this expanded state, it can easily be slipped onto the wooden wheel. Once in place, the rim is cooled down with water. As it cools, it contracts and shrinks back to its original size, creating a very tight, firm grip on the wooden wheel. This ensures the metal tyre is securely fixed.
(c) The gaps are left between the successive rails on a railway track.
Railway tracks are made of steel, which expands in hot weather and contracts in cold weather. If the rails were laid end-to-end without any gaps, this expansion in summer would cause the tracks to buckle and bend out of shape, leading to derailments. The gaps between rails provide the necessary space for the metal to expand into safely when the temperature rises.
(d) A glass stopper stuck in the neck of a bottle can be removed by pouring hot water on the neck of the bottle.
When a glass stopper is stuck, it is often due to a tighter fit. Glass is a poor conductor of heat. Pouring hot water on the neck of the bottle causes the outer glass to heat up and expand. The inner part of the neck, which is in contact with the stopper, expands first and to a greater extent than the stopper itself (as the heat takes time to reach the stopper through the glass). This slight increase in the size of the bottle’s neck creates a little extra space, loosening the stopper and making it easier to remove.
(e) A cement floor is laid in small pieces with gaps in between.
Cement and concrete expand slightly in heat and contract in cold. If a large cement floor were laid as a single, continuous sheet, this expansion and contraction would create immense stress, causing the floor to crack randomly and unevenly. By laying the floor in small tiles or sections with gaps in between (which are often filled with a flexible material), we provide controlled spaces for the sections to expand and contract slightly without causing any major cracks. This keeps the floor stable and prevents damage.
Question 25. Why is one end of a steel girder in a bridge kept on rollers instead of fixing it in pillar ?
Ans: One end of a steel girder in a bridge is kept on rollers instead of being fixed firmly to a pillar to allow for the expansion and contraction of the steel.Steel, like most materials, expands when it gets hot in the summer and contracts when it gets cold in the winter. This change in length is a natural physical process.If both ends of the girder were rigidly fixed to the pillars, this natural movement would have no room to occur. The girder would experience immense stress, pushing or pulling against the pillars with great force. Over time, this would lead to:
Bending or warping of the girder.
Damage to the pillars and their foundations.
Development of cracks in the structure.
The rollers act as a movable support. When the girder expands, it can push the rollers and lengthen slightly without building up dangerous stress. When it contracts, it can pull back. This simple mechanism safely absorbs the thermal expansion, protecting the bridge’s structural integrity and ensuring its long life.
Question 26. A metal plate is heated. State three factors on which the increase in its area will depend.
Ans: The increase in the area of a heated metal plate depends on the following three factors:
Original Area: The expansion is proportional to the initial size. A larger plate will experience a greater total increase in area than a smaller plate made of the same material and heated through the same temperature change.
Rise in Temperature: The higher the temperature to which the plate is heated, the greater the expansion. A plate heated to 200°C will expand more than the same plate heated to only 100°C.
Nature of the Metal (Material): Different metals expand at different rates. This is determined by a property called the coefficient of superficial (area) expansion. For example, aluminium will expand more than iron for the same size and temperature increase.
Question 27. A cubical metal solid block is heated. How will its volume change ?
Ans: When a cubical metal solid block is heated, its volume will increase.This happens because heating provides energy to the metal atoms, causing them to vibrate more vigorously. These stronger vibrations push the atoms slightly further apart from each other, leading to an expansion in all three dimensions—length, breadth, and height. Since volume depends on all three dimensions, the overall volume of the cube increases. This phenomenon is known as thermal expansion.
Question 28. Describe an experiment to show that liquids expand on heating.
Ans:
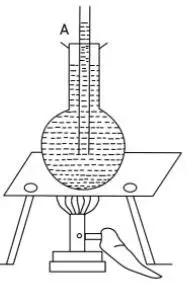
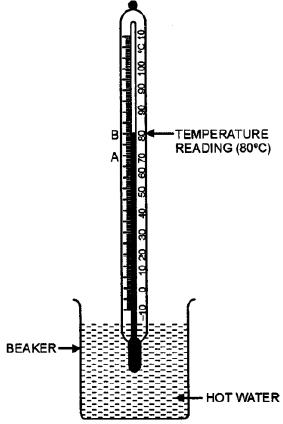
Aim: To demonstrate that liquids expand when heated.
Apparatus Required: A round-bottomed flask, a cork with a narrow hole, a thin glass tube (like a capillary tube), a beaker, hot water, a burner, water, and ink.
Procedure:
Setup: Fill the round-bottomed flask completely with water coloured with a few drops of ink. This makes the water level easily visible.
Insert the Tube: Insert the thin glass tube through the hole in the cork. Push the cork tightly into the mouth of the flask. You will observe that a small amount of coloured water rises up into the thin glass tube. Mark this initial water level in the tube with a pen or a marker. Let this be point A.
Heating: Now, carefully place the flask in a beaker containing hot water. Alternatively, you can gently heat the flask using a burner. Ensure the heat is applied evenly.
Observation: As the flask and the coloured water inside it get heated, you will observe that the level of the coloured water in the thin glass tube starts to rise steadily.
Conclusion: The only explanation for the rising water level is that the water inside the flask is expanding and needs more space. Since the flask is rigid, the expanding water is forced to move up the narrow tube. This proves that liquids, like water, expand on heating.
Precautions:
The flask should be filled completely so that no air is trapped inside.
The cork must be airtight.
Heating should be done gently and evenly.
Question 29. State one application of thermal expansion of liquids.
Ans: A very common and everyday use of the thermal expansion of liquids is the ordinary thermometer.
Here is how it works: Inside the thermometer, there is a thin, hollow glass tube with a small bulb at the bottom filled with a liquid, typically mercury or coloured alcohol. When the thermometer is placed in a warmer environment, the heat causes the liquid inside to absorb energy. This makes the liquid particles move more vigorously and take up more space, forcing the liquid to expand. Because the glass tube is so narrow, this expansion is very noticeable, pushing the liquid column upward.
Conversely, when the thermometer is placed in a cooler place, the liquid loses heat energy. The particles move less and settle closer together, causing the liquid to contract. This makes the level of the liquid drop down the tube. By marking a scale on the glass that corresponds to known temperatures like the freezing and boiling points of water, we can easily tell the temperature just by looking at how high or low the liquid has risen or fallen.
Question 30. Describe an experiment to show that air expands on heating.
Ans:

Observation:
After heating the bottom of the flask for a few minutes, air bubbles are seen escaping from the open end of the glass tube into the water in the beaker.
Explanation:The heat from the candle warms the air trapped inside the flask. This heat energy makes the air molecules move faster and spread apart from each other. Because the molecules are more spread out, the same amount of air now needs more space, or in other words, it expands. Since the flask is inverted and the only exit is through the glass tube, this expanding air is forced out, creating the stream of bubbles that we observe in the water.
Conclusion:This simple demonstration clearly shows that air occupies more space when it is heated.
Question 31. An empty glass bottle is fitted with a narrow tube at its mouth. The open end of the tube is kept in a beaker containing water. When the bottle is heated, bubbles of air are seen escaping into water. Explain the reason.
Ans: When the bottle is heated, the air trapped inside the bottle and the tube gets warmed up. As we know, heating a gas causes it to expand. The expanding air needs more space, so it pushes its way out through the narrow tube. This is why we see bubbles of air escaping into the water in the beaker.Once the bottle is removed from the heat, the air inside cools down and contracts (takes up less space). This creates a partial vacuum or low pressure inside the bottle. The higher atmospheric pressure outside now pushes the water from the beaker up into the tube to fill the empty space.
Question 32. Which of the following will expand more, when heated to the same temperature : (a) solid (b) liquid and (c) gas ?
Ans: When heated equally, a gas expands the most, a liquid expands less than a gas, and a solid expands the least.This happens because the particles in a gas are far apart and move freely, so heat makes them move much farther. Liquid particles can slide past each other and need more space when heated, but not as much as a gas. Solid particles are tightly packed in a fixed structure; they only vibrate more when heated, leading to a very small expansion.
So, the order is: Gas > Liquid > Solid.
Question 33. Describe an experiment to show that same volume of different liquids heated to same rise in temperature expand by different amounts.
Ans:
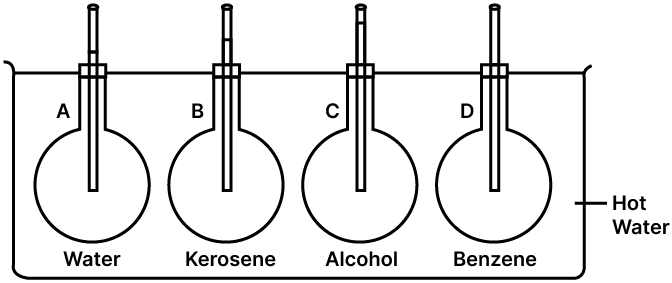
Aim:
To demonstrate that an equal volume of different liquids expands by a different amount when heated through the same rise in temperature.
Apparatus:
You will need three or four identical round-bottomed flasks, corks with holes, long thin glass tubes, a large beaker, different coloured liquids (such as water, kerosene, and ethanol), a source of heat (like a heater), a ruler, and a thermometer.
Procedure:
Take the identical flasks and fill each one with the same volume of a different liquid (e.g., water in one, kerosene in another, ethanol in a third).
Fit a cork into the mouth of each flask. A long, narrow glass tube should pass through each cork down into the liquid.
At the start, the liquid level will be the same in all the tubes. Mark this initial level with a permanent marker.
Now, place all the flasks into the same large beaker filled with hot water, or arrange them so they are all heated equally by the same heater. This ensures all the liquids experience the same temperature increase.
Allow the flasks to heat for a few minutes and carefully observe the liquid levels in the glass tubes.
Observation:
After heating, you will notice that the liquid level has risen in all the glass tubes. However, the final level in each tube is different. For instance, the level of ethanol will be the highest, followed by kerosene, and then water. This shows that each liquid expanded by a different amount.
Conclusion:
This experiment clearly shows that even when the same initial volume of different liquids is heated equally, they do not expand by the same amount. This happens because each liquid has its own unique coefficient of expansion, which determines how much its volume increases with temperature.
Question 34. 100 ml of each of the following liquid is heated from 10°C to 50°C. Which will expand more : (a) water (b) benzene (c) alcohol ?
Ans: Of the three liquids—water, benzene, and alcohol—benzene will expand the most when heated from 10 °C to 50 °C.
Here’s why:
Liquids expand at different rates when heated, and this is measured by their coefficient of volume expansion.
Benzene has a high coefficient of volume expansion (about 1.24×10 −3 / ∘ C), meaning it expands significantly with temperature increase.
Alcohol (ethyl alcohol) also expands more than water (about 1.12×10 −3 / ∘ C), but less than benzene.
Water behaves unusually: between 0 °C and 4 °C it actually contracts on heating. From 10 °C to 50 °C it does expand, but its expansion coefficient (around 0.21×10 −3 / ∘ C in the 20–50 °C range) is much lower than that of benzene and alcohol.
So the order of expansion (most to least) is:
Benzene > Alcohol > Water.
Question 35. Water is heated from 0°C to 4°C. Will it expand ?
Ans: No, water does not expand when heated from 0°C to 4°C. It actually contracts.
This is a unique behaviour of water known as anomalous expansion. Water has its maximum density at 4°C. So, when ice-cold water at 0°C is warmed, its volume decreases and it becomes denser until it reaches 4°C. Only above 4°C does water begin to expand normally upon heating.
Question 36. What do you mean by anomalous behaviour of water ?
Ans: The anomalous behaviour of water refers to its unique and exceptional property of expanding when cooled below 4°C.
Normally, most liquids contract continuously on cooling.
However, water contracts and becomes denser only until 4°C.
When cooled further from 4°C to 0°C, it expands and becomes less dense.
This is why ice (solid water) is less dense than liquid water and floats on it. This anomaly is crucial for the survival of aquatic life in winters.
Question 37. How does the density of a substance (solid, liquid and gas) change on heating ?
Ans: On heating, the density of a substance decreases for all three states: solid, liquid, and gas.
This happens because when a substance is heated, it gains energy and its particles vibrate or move more vigorously. This increased movement causes the substance to expand, meaning its volume increases.
Since Density = Mass / Volume and the mass remains constant, an increase in volume leads to a decrease in density.
Solids: Expand the least, so the decrease in density is small.
Liquids: Expand more than solids, so the density decreases more noticeably.
Gases: Expand the most dramatically, causing the density to decrease very significantly upon heating.
Question 38. An iron washer is heated. State the effect on its (i) mass, (ii) internal diameter, (iii) external diameter, and (iv) density.
Ans: (i) Mass:
The mass of the washer will stay the same. This is because heating it does not add or take away any of the actual material the washer is made from. The number of atoms in it remains constant.
(ii) Internal Diameter:
The internal diameter, or the size of the hole, will get larger. Many people think the hole would shrink, but the entire piece of metal expands outwards, making the hole bigger as well.
(iii) External Diameter:
The external diameter will increase. When heated, the metal expands in all directions, so the overall outside size of the washer grows.
(iv) Density:
The density of the washer will decrease. Density is mass divided by volume. Since the mass is unchanged but the washer’s volume has increased, the same amount of mass is now spread out in a larger space, making it less dense.