Water is fundamentally the solvent of life, forming the base of all biological processes. Its unique physical and chemical properties, like a high specific heat capacity, high latent heat of vaporization, and the unusual property of becoming less dense as a solid (ice), make it indispensable. These characteristics are crucial for sustaining life. For instance, water’s high heat capacity allows it to absorb a lot of heat without a significant rise in temperature, which helps organisms maintain a stable internal body temperature and prevents large fluctuations in environmental temperatures. Furthermore, its expansion upon freezing means ice floats, insulating the water below and allowing aquatic life to survive in colder climates.
The chapter then delves into the sources of water and the critical issue of water pollution. It explains the natural water cycle, which continuously replenishes our freshwater sources like rivers, lakes, and groundwater. However, human activities severely threaten this supply. Pollution is introduced through various means, including industrial waste discharged directly into water bodies, agricultural runoff containing chemical fertilizers and pesticides, and domestic sewage. This contamination not only makes water unfit for human consumption but also disrupts aquatic ecosystems, leading to problems like eutrophication, where excess nutrients cause algal blooms that deplete oxygen and kill fish.
Finally, the chapter emphasizes the vital need for water conservation and the methods to achieve it. With a growing global population and increasing industrialization, the demand for clean water is escalating, making its prudent management essential. The text discusses both collective and individual measures for conservation. These include large-scale projects like building dams and watershed management, as well as everyday practices such as fixing leaking taps, practicing rainwater harvesting, and using water-efficient appliances. The underlying message is that water is a precious, finite resource, and its preservation is a shared responsibility for the survival of our planet and future generations.
Exercise 3 (A)
Question 1.
Water exists in all three states. Discuss.
Ans:
1. The Liquid: The State of Life
This is the water we are most familiar with. We drink it, wash with it, and it fills our rivers, lakes, and oceans. Water is a liquid at the temperatures and pressures commonly found on Earth’s surface. This liquid state is vital because it acts as a universal solvent, allowing essential nutrients and minerals to dissolve and be transported through the bloodstream of animals and the sap of plants. The very chemistry of life occurs in watery solutions.
2. The Solid: The Floating State
When the temperature drops to 0°C (32°F) or below, liquid water freezes into a solid—ice. What’s truly fascinating and unique about water’s solid state is that it is less dense than its liquid form. This is why ice cubes float in your glass. For almost every other substance, the solid state is denser and sinks.
This peculiar property has profound consequences. In nature, when a lake freezes, the ice forms a layer on the surface. This layer acts as an insulator, preventing the water below from freezing solid. This allows fish and other aquatic life to survive the winter. Without this simple fact, many bodies of water would freeze from the bottom up, making aquatic life in temperate climates nearly impossible.
We see water’s solid state in glaciers, snowflakes, hail, and the frost on a windowpane.
3. The Gas: The Invisible Traveller
Water is constantly on the move, and its gaseous state is how it travels. Through a process called evaporation, molecules of liquid water at the surface gain enough energy to escape into the air as an invisible gas called water vapor. This happens all the time, from puddles drying up after a rainstorm to the transpiration from the leaves of plants.
This gaseous water is a key component of our atmosphere and is the driving force behind the planet’s water cycle. When this vapor rises and cools, it condenses back into tiny liquid droplets, forming clouds. Eventually, these droplets coalesce and fall back to Earth as precipitation (rain, snow, sleet), replenishing the liquid and solid states.
We can’t see water vapor, but we feel it as humidity. And we see its effects when it condenses on a cold drink on a hot day or when our breath turns to a mist in chilly air.
The Driving Force Behind the Change
The reason water can so easily shift between these three states is due to the energy in its environment.
- Adding Energy (Heat): When you add heat to ice, it melts into liquid water. Add more heat, and the liquid evaporates into water vapor. This is why a puddle disappears on a sunny day.
- Removing Energy (Cooling): When water vapor cools, it condenses into liquid. When liquid water loses enough heat energy, it freezes into solid ice.
Question 2.
Why is water considered a compound?
Ans:
Water is classified as a compound because it is a substance formed when two different elements, hydrogen and oxygen, are chemically bonded together in a fixed proportion. It is not just a simple mixture; its creation involves a chemical reaction that fundamentally changes the original properties of the elements involved. For instance, the hydrogen and oxygen that combine to form water are both gases at room temperature, but the result of their union is a liquid with entirely different characteristics. This transformation is a classic sign of a compound being formed, where the constituent elements lose their individual identities to create a new substance with its own unique set of properties.
The defining evidence for water being a compound lies in its fixed composition. A molecule of water is always made up of two hydrogen atoms bonded to one oxygen atom, giving it the constant chemical formula H₂O. This consistent ratio of 1:8 by mass of hydrogen to oxygen is a fundamental law of chemical combination and cannot be altered without creating a different substance altogether. Unlike a mixture, where components can be mixed in any proportion and often separated by simple physical means, the elements in water cannot be separated by filtration or evaporation and can only be divided through a chemical process like electrolysis. This unchangeable atomic structure and the energy changes involved in its formation and decomposition firmly establish water’s identity as a pure chemical compound.
Question 3.
1. Why does the temperature in Mumbai and Chennai not fall as low as it does in Delhi? 2. Give the properties of water responsible for controlling the temperature of our body.
Ans:
1. Why does the temperature in Mumbai and Chennai not fall as low as it does in Delhi?
The key reason for this difference lies in the moderating influence of the sea, a phenomenon tied to water’s high specific heat capacity. Coastal cities like Mumbai and Chennai are located near the Arabian Sea and the Bay of Bengal respectively. Because water heats up and cools down much more slowly than land, these coastal areas experience milder temperatures throughout the year. The sea acts like a giant temperature buffer, absorbing heat during the summer and releasing it slowly during the winter, which prevents the sharp drop in temperature that inland cities experience.
In contrast, Delhi is a landlocked city located far from any major sea. Land has a lower specific heat capacity, meaning it gains and loses heat very rapidly. During the winter, the land in and around Delhi cools down significantly and quickly, leading to much colder temperatures, especially at night. Furthermore, cold winds from the snow-covered Himalayas sweep across the northern plains, contributing to the intense winter chill in Delhi, an effect that does not reach the coastal regions.
2. Give the properties of water responsible for controlling the temperature of our body.
Our bodies rely heavily on two specific properties of water to maintain a stable internal temperature: its high specific heat capacity and its high latent heat of vaporization.
First, water’s high specific heat capacity means it can absorb a large amount of heat energy without its own temperature rising dramatically. A major component of our body is water, which allows it to act as a heat reservoir. This property ensures that during physical activity or in a warm environment, the heat generated within our body does not cause a sudden, dangerous spike in our core temperature. The body’s water content absorbs this excess heat, helping to keep our temperature stable.
Second, the process of sweating utilizes water’s high latent heat of vaporization. This is the significant amount of heat energy water requires to change from a liquid to a gas. When our body needs to cool down, it secretes sweat onto the skin. As this sweat evaporates, it draws a substantial amount of heat from the skin surface, providing a powerful cooling effect. This efficient mechanism prevents us from overheating and is crucial for survival, especially in hot conditions or during exertion.
Question 4.
‘Water is the universal solvent’. Comment.
Ans:
The declaration that “water is the universal solvent” is a powerful, yet subtly misleading, piece of scientific shorthand. It is less an absolute truth and more a testament to water’s unparalleled promiscuity in interacting with other substances. To call it “universal” is not to say it dissolves everything—a glance at a greasy oil slick on a puddle proves it does not. Instead, the label honors its exceptional capacity to dismantle a wider range of materials than any other liquid.
This remarkable ability is rooted in the quirky, lopsided architecture of the water molecule itself. Its bent shape, with oxygen hogging the electrons, creates a strong polarity: a slightly negative oxygen end and slightly positive hydrogen ends. This makes water a molecular magnet. When a pinch of salt (sodium chloride) is introduced, the water molecules swarm it. The negative oxygen ends latch onto positive sodium ions, while the positive hydrogen ends surround negative chloride ions, prying them away from their crystalline lattice. This “solvation” process is water’s signature move.
However, water’s limitations are as telling as its powers. It is famously inept at dissolving nonpolar substances like oils and waxes. The phrase “like dissolves like” is the crucial corollary to its “universal” title. Water cannot form bonds with these molecules, so it pushes them away, forcing them to clump together. This very failure is the foundation of cell membranes, which rely on this repulsion to form protective barriers. Life, paradoxically, depends as much on what water cannot dissolve as on what it can.
Furthermore, water’s solvency is a double-edged sword. Its eagerness to dissolve gases from the air, like oxygen, allows aquatic life to breathe. Yet, this same property makes it a vulnerable carrier of pollution. It readily dissolves agricultural runoff and industrial waste, becoming a vector for contaminants. Its “universal” nature is what makes it both the cradle of life and a potential vehicle for its harm.
Question 5.
What causes the violence associated with torrential rain?
Ans:
1. The Onslaught of Hydrological Overload
At its core, the violence begins with water’s simple, immutable properties, but on a catastrophic scale.
- The Runoff Hammer: When rain falls at an exceptional rate—often dozens of millimeters per hour—the ground cannot absorb it. Soil becomes saturated almost instantly, acting like an impermeable sheet. This creates an immense, sudden volume of surface runoff. This isn’t a gentle flow; it’s a collective surge of water that sheets across the land, gathering speed and power as it funnels into dips and channels. This runoff doesn’t just flow; it scrapes, scours, and dismantles the surface layer of the earth.
- The Scour and Saturation of Soil: This deluge violently separates soil particles, breaking the root structures of plants and grasses that hold the earth together. The soil loses its cohesion and becomes suspended in the water, transforming it into a dense, abrasive fluid. This process of saturation is what primes slopes for catastrophic failure, setting the stage for landslides.
2. The Rampage of Rushing Water: Kinetic Energy Unleashed
Water in motion possesses kinetic energy, which increases with the square of its velocity. This physical principle is key to understanding the destruction.
- The Debris-Fueled Torrent: As the initial surge of runoff collects in streams and gullies, it does not travel empty-handed. It plucks at everything in its path: loose soil, rocks, branches, and human debris like fences and signage. This mixture creates a thick, churning slurry of mud and rock. This debris load is not just carried by the water; it becomes a primary tool of destruction. The rocks and boulders within the flow act like battering rams, slamming into structures, bridge pylons, and riverbanks with devastating effect.
- The Erosive Carve: The abrasive quality of this debris-laden water acts like liquid sandpaper on a monumental scale. It can gouge out riverbanks, undercut foundations, and dig channels meters deep in a matter of hours, destabilizing everything above. This erosion is often silent and out of sight until the moment of collapse.
3. The Collapse of the Foundation: Mass Wasting
When the soaked, heavy earth can no longer resist gravity, the violence shifts from flowing to falling.
- The Liquefaction of Slopes: On steep terrain, the rainwater doesn’t just rest in the soil; it fills the pores between particles, dramatically increasing the weight and pressure of the slope. The water pressure pushes the soil grains apart, reducing friction. In this state, the solid ground effectively turns into a liquid, a process known as liquefaction. This transforms a stable hillside into a mobile, semi-fluid mass.
- The Landslide Avalanche: This mobilized earth then detaches and accelerates downhill. This is not a slow creep but a sudden, avalanche-like flow of mud, rock, trees, and debris. It can travel at high speeds, obliterating everything in its path with immense, smothering force. The violence here is one of crushing weight and burial, often with little warning.
4. The Choke and Surge of River Systems
The violence is often concentrated and amplified where water is naturally channeled.
- The Channel Blockage: Intense runoff and small landslides often carry large woody debris—trees, logs, and vegetation—into river channels. These materials can accumulate at narrow points, such as bridges or culverts, forming a temporary dam. This “debris jam” backs up water, creating a growing reservoir of pent-up energy behind it.
- The Catastrophic Breach: The makeshift dam, unable to withstand the rising pressure, eventually fails catastrophically and all at once. This releases the stored water in a powerful, sudden wave—a mini-tsunami—that roars downstream. This wave carries the destructive force of the initial debris jam plus all the new water and debris it picks up, leading to explosive flooding downstream of the blockage.
Question 6.
1. Which property of water enables it to modify the climate?
2. The density of water varies with temperature. What are its consequences?
3. What is the effect of impurities present in the water on the melting point and boiling point of water?
Ans:
1. The property of water responsible for modifying climate is its high specific heat capacity. This means water needs to absorb a considerable amount of heat energy to raise its temperature, just as it must release a significant amount of energy to cool down. This characteristic acts as a global temperature buffer. Large water bodies like oceans and lakes absorb solar heat during the day and summer months without a drastic rise in their own temperature, and then they slowly release this stored heat during the colder nights and winter. This process moderates temperature extremes, leading to milder and more stable climates in coastal regions compared to the harsh extremes found in inland deserts.
2. The variation of water’s density with temperature has a crucial and unusual consequence, most notably that solid water (ice) is less dense than liquid water. Unlike most substances, which become denser as they solidify, water reaches its maximum density at 4°C and expands as it freezes into ice at 0°C. This is why ice cubes float in a glass. In nature, this phenomenon is vital for aquatic life. During winter, a layer of ice forms on the surface of lakes and ponds, and because it floats, it acts as an insulating blanket. This prevents the entire water body from freezing solid, allowing fish and other organisms to survive in the liquid water below the ice.
3. The presence of impurities, such as dissolved salts or other substances, directly affects the phase change points of water. Impurities cause a lowering of the melting point and an elevation of the boiling point, an effect known as colligative properties. For example, when salt is sprinkled on icy roads, it disrupts the water’s ability to form a solid lattice, forcing it to remain liquid at temperatures below 0°C, which is why the ice melts. Conversely, in the kitchen, it is observed that adding salt to water makes it boil at a temperature slightly higher than 100°C. This is because the impurities interfere with the molecules’ ability to escape into the vapor phase, requiring more energy (and thus a higher temperature) to boil.
Question 7.
How do fishes and aquatic animals survive when the pond gets covered with thick ice?
Ans:
The formation of a frozen layer on a pond’s surface is a lifesaving event for the ecosystem within. This happens because of a special property of water: it expands as it turns solid, making ice lighter than the liquid beneath it. Instead of sinking, this frozen cap settles on top, creating a protective barrier against the harsh outside air. This layer effectively seals in the water’s residual warmth, stopping the body of water from freezing solid from the bottom up. The pond’s depths remain a liquid refuge, insulated by the very ice that covers it.
For the fish living in this chilly world, their entire way of life slows to a crawl. Their body temperature drops to match the cold water, causing their metabolism to plummet. They enter a state of profound lethargy, conserving every bit of energy. Movement is minimal, and bodily functions like digestion and heart rate become incredibly slow. They often hunker down in the slightly warmer mud and sediments at the bottom, waiting out the winter in a dormant state that is not unlike hibernation.
Adding to this delicate balance, the icy ceiling allows sunlight to filter through, especially if the ice is clear. This light is a crucial energy source for submerged aquatic vegetation. These plants can continue to perform a subdued level of photosynthesis beneath the ice. While not as vigorous as in summer, this process generates a small, steady trickle of oxygen, dissolving directly into the water. This continuous supply is just enough for the dormant creatures, allowing them to breathe until the spring thaw returns.
Question 8.
The properties of water are different from the properties of the elements of which it is formed. Discuss.
Ans:
Water is a compound, and its properties are completely different from the hydrogen and oxygen that create it. This dramatic change occurs because during chemical bonding, atoms lose their original identities and form a new substance with a unique structure.
For example, the two elements that form water are:
- Hydrogen (H₂): A highly flammable, lightweight gas.
- Oxygen (O₂): A gas that vigorously supports combustion.
When these two gases chemically combine (H₂ + O), they form water (H₂O), which is a liquid that actually extinguishes fire. This new substance has emergent properties—like being a universal solvent, having high surface tension, and existing as a liquid at room temperature—that are not just a blend of its gaseous components but are entirely new behaviors resulting from their chemical union.
Question 9.
How is aquatic life benefited by the fact that water has maximum density at 4oC?
Ans:
Aquatic life benefits tremendously from water’s unique property of reaching its maximum density at 4°C, rather than at its freezing point. This behaviour is a fascinating exception in nature. As water cools towards 4°C, it becomes heavier and sinks. However, once it cools further below 4°C, it becomes less dense and starts to expand. This is why ice, being lighter than liquid water, forms on the surface of a lake or pond first.
This simple physical quirk creates a life-saving insulation effect. The layer of ice that forms on the surface acts as a barrier, trapping the relatively warmer water (at around 4°C) beneath it. This prevents the entire body of water from freezing solid from the bottom up. Consequently, even during the harshest winters, a stable, liquid environment is maintained at the bottom of lakes and ponds.
Within this underwater refuge, aquatic organisms like fish, insects, amphibians, and plants can survive the freezing temperatures above. They are protected from being frozen and have access to liquid water, which is essential for their biological processes. Without this peculiar density rule, bodies of water would freeze completely in cold climates, making it impossible for most aquatic ecosystems to exist as we know them.
Question 10.
What are the observations and conclusions when tap water is boiled and evaporated in watch glass?
Ans:
Experiment: Boiling Tap Water and Evaporating it in a Watch Glass
Aim: To analyze the residue left after the evaporation of tap water and draw conclusions about its purity.
Procedure: A small amount of tap water is boiled vigorously for a minute in a beaker. While still hot, a portion of this water is transferred to a clean, dry watch glass. The watch glass is then left in a safe, warm place (like on a hotplate set to low or in a sunny spot) for the water to evaporate completely.
Observations
During the boiling and evaporation process, the following would be observed:
1. During Boiling:
- As the water is heated, tiny bubbles of dissolved air begin to form on the inner surface of the beaker and then rise to the top.
- Upon reaching boiling point, rapid, continuous bubbling is seen throughout the liquid as water vapor is produced.
- A faint, white precipitate may become visible in the boiling water, especially in areas with “hard” water.
2. On the Hot Watch Glass:
- The water initially forms a clear, shallow pool.
- As it begins to cool, it remains clear for a short period.
3. During Evaporation:
- The volume of water on the watch glass gradually decreases over time.
- As the water volume becomes very small, a distinct white, chalky ring starts to form at the edges where the water meniscus was located.
- The very last droplets of water may appear slightly cloudy before they completely disappear.
4. After Complete Evaporation:
- The initially clean and transparent watch glass is no longer clear.
- A solid, white, scaly or powdery residue is left behind, coating the bottom of the watch glass.
- This residue is often crystalline in appearance under magnification and may form circular patterns corresponding to the final movement of the evaporating water.
Conclusions
Based on these observations, we can draw the following conclusions:
- Tap Water is a Mixture, Not a Pure Substance: The fact that a solid residue remains after the water (a liquid) has vanished proves that tap water is not pure. It is a homogeneous mixture (a solution) of water and various dissolved solids.
- Presence of Dissolved Salts and Minerals: The white residue is clear evidence of non-volatile dissolved substances. These are primarily inorganic salts. Common compounds include:
- Calcium Carbonate (CaCO₃): The primary component of the white scale, especially formed when temporary hardness (calcium bicarbonate) in water decomposes upon heating.
- Magnesium Salts, and possibly Sulfates and Chlorides of calcium and magnesium.
- Explanation of the Boiling Process: The initial tiny bubbles observed were not steam but dissolved air being driven out. The continuous bubbling at 100°C confirmed the water was boiling and turning into vapor. The potential formation of a precipitate during boiling is due to the thermal decomposition of soluble calcium bicarbonate into insoluble calcium carbonate, which is the same compound that makes up the final residue.
- The Ring Formation: The reason the residue forms a distinct ring is that as evaporation occurs at the edges, the water from the center flows outward to replace it through capillary action. This flow carries dissolved minerals to the edge, where they are deposited as the water evaporates, concentrating the solids in that ring-shaped zone.
- Practical Implication: This simple experiment demonstrates the cause of “scale” or “limescale” in kettles, water heaters, and pipes. The dissolved minerals in tap water precipitate out upon heating, forming a hard, insulating layer that can reduce the efficiency of appliances and clog plumbing over time. It visually confirms why we often treat or filter water for certain uses.
Question 11.
What is the importance of dissolved salts in water?
Ans:
The presence of dissolved salts transforms plain water into a vital, life-supporting medium. These salts, which include essential minerals like sodium, potassium, calcium, magnesium, and chlorides, are not just impurities but are fundamental to the survival of both plants and animals. In our bodies, for instance, these salts act as electrolytes. They play a starring role in maintaining the delicate fluid balance within and around our cells, ensuring that nerves can transmit signals effectively and that our muscles, including the heart, can contract properly. Without this specific blend of salts in our bodily fluids, these critical processes would simply fail.
For plants, the importance is equally profound. They absorb these essential mineral nutrients directly from the soil water. Salts like nitrates and phosphates are the building blocks for creating proteins and nucleic acids, which are the very foundation of plant growth and development. Magnesium is a central component of chlorophyll, without which photosynthesis cannot occur. Furthermore, these dissolved salts influence the osmotic balance, regulating how water moves in and out of plant cells. If the concentration is wrong, plants can either wilt from water loss or burst from excessive water intake.
Beyond biology, dissolved salts have a significant impact on the physical properties of water itself. A key example is that they lower the freezing point of water, a phenomenon we see with seawater that remains liquid at temperatures where freshwater would turn to ice. They also increase the density and electrical conductivity of water. This conductivity is what allows certain aquatic creatures, like some species of sharks and eels, to sense their surroundings through electroreception. In essence, these dissolved salts make water a more dynamic and functional substance, enabling the complex chemistry that underpins all known life.
Question 12.
State the importance of the solubility of CO2 and O2 in water.
Ans:
The solubility of both oxygen (O2) and carbon dioxide (CO2) in water is a cornerstone of aquatic life and global ecological balance. While neither gas is highly soluble, the fact that they do dissolve to some extent is what makes life in water possible. Oxygen’s ability to dissolve is directly responsible for supporting the respiration of all aerobic aquatic organisms, from fish and insects to bacteria. These creatures rely on the oxygen present in the water, which primarily enters through diffusion at the surface and from aquatic plant photosynthesis, to break down their food and release energy for survival. Without this dissolved oxygen, rivers, lakes, and oceans would be lifeless, anaerobic environments.
On the other hand, the solubility of carbon dioxide holds a different but equally critical set of importances. CO2 dissolves much more readily in water than oxygen, and this property is fundamental for aquatic photosynthesis. Aquatic plants and phytoplankton, which form the base of most aquatic food webs, use dissolved carbon dioxide to produce their own food and release oxygen back into the water. Furthermore, when CO2 dissolves, it forms weak carbonic acid, which plays a vital role in shaping the environment. In the oceans, this reaction allows for the formation of carbonate and bicarbonate ions, which are the essential building blocks that marine organisms like corals, mollusks, and some plankton use to create their shells and skeletons. Therefore, the solubility of CO2 directly governs the health of entire marine ecosystems and the global carbon cycle.
Question 13.
How is air dissolved in water different from ordinary air?
Ans:
1. The Pressure and Solubility Relationship
The most critical difference lies in how the gases exist. Ordinary air is a free mixture of gases, with each molecule whizzing around independently. In water, these gas molecules are not free; they are dissolved, forced by the pressure of the atmosphere above the water to become trapped between the water molecules.
This leads to a crucial distinction: the composition is skewed. In ordinary air, nitrogen (N₂) makes up about 78% and oxygen (O₂) about 21%. However, oxygen is more soluble in water than nitrogen. As a result, in dissolved air, the percentage of oxygen is much higher relative to nitrogen. While the exact ratio depends on temperature and pressure, dissolved air can be over 30% oxygen by volume of the gas released, making it a much more oxygen-rich mixture than the air around us.
2. The Impact of Temperature
Temperature plays a minor role in the behavior of ordinary air in our daily lives, but it is a dominant force for dissolved air. Cold water can hold significantly more dissolved gas than warm water. This is why a cold glass of water left on a counter will slowly form tiny bubbles on its sides as it warms up—the water is releasing its dissolved air because its capacity to hold the gas has decreased.
3. Biological and Chemical Activity
Ordinary air is relatively inert in its gaseous state. Dissolved air, particularly the oxygen within it, is the engine for aquatic life. Fish and other organisms don’t breathe ordinary air; they extract this dissolved oxygen directly from the water through their gills. Furthermore, dissolved oxygen is a key player in chemical processes, such as the rusting of iron underwater (corrosion) and the natural breakdown of organic waste in lakes and rivers.
4. Behavior and State of Matter
This is a fundamental physical difference. Ordinary air is a gas. The dissolved air in water is not a separate phase; it’s a solute in a liquid solvent. You cannot separate them by filtration, only by changing the conditions, like heating the water or reducing the pressure (which is how bubbles form when you open a carbonated drink—the pressure is released, and the dissolved CO₂ comes out of solution).
Composition Differences
| Component | Ordinary Atmospheric Air (Gas Phase) | Air Dissolved in Water (Aqueous Phase) |
| Nitrogen (N2) | Highest (approx. 78%) | Lower concentration relative to oxygen. |
| Oxygen (O2) | Second highest (approx. 21%) | Higher concentration relative to nitrogen. |
| Reason for Difference | Nitrogen is less soluble in water than oxygen is. | Oxygen is nearly twice as soluble in water as nitrogen is, leading to a much higher proportion of oxygen in the dissolved air mix. |
Question 14.
Identify A, B, C and D; the first one is done for you.
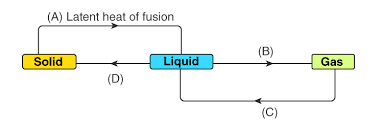
Ans:
Based on the provided diagram illustrating the phase changes between the solid, liquid, and gas states, here are the terms represented by A, B, C, and D:
| Label | Phase Change | Term |
| (A) | Solid → Liquid | Latent heat of fusion (or Melting/Fusion) |
| (B) | Liquid → Gas | Latent heat of vaporization (or Vaporization/Boiling) |
| (C) | Gas → Liquid | Condensation |
| (D) | Liquid → Solid | Freezing (or Solidification) |
The full process involves:
- A (Fusion/Melting): Absorption of latent heat to change solid to liquid.
- B (Vaporization): Absorption of latent heat to change liquid to gas.
- C (Condensation): Release of heat to change gas back to liquid.
- D (Freezing/Solidification): Release of heat to change liquid back to solid.
Question 15.
1. Explain why boiled or distilled water tastes flat.
2. Explain why: Ice at zero degrees centigrade has a greater cooling effect than water at 0oC.
3. Explain why: Burns caused by steam are more severe than burns caused by boiling water.
4. Explain why rivers and lakes do not freeze easily.
5. Explain why: Air dissolved in water contains a higher proportion of oxygen.
6. Explain why: If distilled water is kept in a sealed bottle for a long time, it leaves etchings on the surface of the glass.
7. Explain why: Rain water does not leave behind concentric rings when boiled.
Ans:
1. Why does boiled or distilled water taste flat?
The distinct taste of regular tap or groundwater isn’t from pure water itself, but from the dissolved gases and minerals present in it, such as oxygen, carbon dioxide, calcium, and magnesium salts. When water is boiled or distilled, these dissolved gases, especially oxygen and carbon dioxide, are driven out. The removal of carbon dioxide is particularly significant as it would normally form a weak carbonic acid, giving water a slightly sharper taste. Without these dissolved substances, the water loses its characteristic taste and feels bland or “flat” on the tongue.
2. Why ice at zero degrees centigrade has a greater cooling effect than water at 0°C.
This is due to the concept of latent heat. Both ice and water at 0°C are at the same temperature, but ice possesses additional hidden energy known as the latent heat of fusion. When ice is used for cooling, it doesn’t just warm up; it first must melt and change into water at 0°C. This melting process requires a substantial amount of energy (80 calories per gram) which it absorbs from its surroundings, like a beverage or your skin. Water at 0°C, on the other hand, only absorbs a small amount of energy to raise its own temperature. Therefore, ice removes significantly more heat from its surroundings to accomplish its phase change, resulting in a much greater cooling effect.
3. Why are burns caused by steam more severe than burns caused by boiling water?
This phenomenon is also explained by latent heat. Boiling water at 100°C carries a certain amount of heat energy. However, when steam at the same temperature condenses on your skin to become water, it releases a large amount of hidden energy called the latent heat of vaporization (a much larger 540 calories per gram). This means a steam burn delivers not only the heat from cooling from 100°C but also this massive extra burst of energy during condensation. Boiling water only transfers the heat from its cooling process, making a steam burn far more severe and damaging to the tissues.
4. Why rivers and lakes do not freeze easily.
This is a consequence of water’s unique property of having maximum density at 4°C. In winter, as the surface water cools, it becomes denser and sinks, pushing the warmer water upward. This process of convection continues until the entire water body reaches 4°C. Only then does the surface water, upon cooling further to 0°C, become less dense and form ice. This ice layer then acts as an insulator, preventing the heat from the deeper, slightly warmer water (at 4°C) from escaping rapidly. This entire process requires a large and prolonged loss of heat, which is why large water bodies do not freeze solid easily or instantly.
5. Why air dissolved in water contains a higher proportion of oxygen.
While the atmosphere is mostly nitrogen (about 78%), the composition of dissolved air in water is different due to the varying solubility of gases. Oxygen is physically more soluble in water than nitrogen. This means that for the same volume of water, a greater proportion of oxygen molecules from the air can dissolve compared to nitrogen molecules. Therefore, even though the air above the water is nitrogen-rich, the dissolved air within the water becomes relatively enriched with oxygen, which is crucial for the survival of aquatic animals like fish.
6. Why distilled water kept in a sealed bottle leaves etchings on the glass.
Distilled water is exceptionally pure and hungry for dissolved substances. Ordinary glass is not completely inert; it contains soluble minerals, primarily sodium and calcium salts. Over a long period, even in a sealed bottle, the highly pure distilled water slowly dissolves or leaches out these minute mineral components from the inner surface of the glass. This very slow chemical erosion creates microscopic pits and grooves, which we see as faint, cloudy markings or “etchings” on the glass surface.
7. Why rainwater does not leave concentric rings when boiled.
The concentric rings, or scale, left behind when hard water is boiled are deposits of dissolved calcium and magnesium salts (like bicarbonates) that precipitate out as carbonates upon heating. Rainwater, in its purest form, is naturally soft. It is formed by the condensation of water vapor and is essentially distilled water, containing very minimal amounts of these dissolved salts. Since there are no significant minerals present to precipitate out, boiling rainwater simply turns it into vapor, leaving behind no visible residue or rings.
Exercise 3 (B)
Question 1.
1. Explain the terms: Solution
2. Explain the term: Solute
3. Explain the terms: Solvent
Ans:
1. Solution
In the simplest terms, a solution is the final, uniform mixture that results when one substance is completely dissolved into another. Think of it as the end product. The key characteristic of a solution is that it is homogeneous, meaning its composition is identical throughout any given sample. You cannot distinguish the individual components by sight, as they are mixed at the molecular or ionic level.
A common real-world example is seawater. When you look at a glass of seawater, you don’t see separate particles of salt floating around; the salt has fully integrated with the water, creating a single, consistent liquid. Other examples include the sugar completely dissolved in your morning coffee, or the carbon dioxide gas dissolved in a bottle of soda. Solutions are not limited to liquids; air, for instance, is a homogeneous solution of primarily nitrogen and oxygen gases.
2. Solute
The solute is the substance that gets dissolved. It is typically the component that is present in a lesser quantity within the solution. The solute undergoes a physical change as it breaks down from its original form (like a sugar crystal or a salt grain) into much smaller particles that disperse and fit into the spaces between the molecules of the solvent.
Using the example of sweet tea, the sugar you stir into the hot tea is the solute. It starts as solid granules but disappears as it dissolves. In a saline solution for contact lenses, the salt is the solute. In the air we breathe, trace gases like carbon dioxide and argon act as solutes within the larger mixture.
3. Solvent
The solvent is the dissolving agent—the substance that does the dissolving. It is almost always the component present in the greatest quantity, acting as the medium or the “host” that accommodates the solute particles. The solvent’s molecular structure is what allows it to surround and isolate the solute particles, preventing them from clumping back together.
In most everyday situations, water is the universal solvent, which is why it features in so many examples. In a mixture of brass, which is an alloy, zinc is the solute dissolved into the larger quantity of copper, which acts as the solvent. Even in a simple act of using rubbing alcohol, the alcohol liquid itself is the solvent that dissolves oils or other contaminants from your skin.
Question 2.
Explain why a hot saturated solution of potassium nitrate forms crystals as it cools.
Ans:
The Driving Force: Changing Solubility
At the heart of this phenomenon is a simple but powerful principle: the solubility of most solid substances is directly tied to the temperature of the solvent. For a salt like potassium nitrate (KNO₃), solubility increases significantly as the temperature of the water rises. This means that hot water can dissolve far more potassium nitrate powder than the same amount of cold water can.
A Step-by-Step Journey of Crystallization
Step 1: Creating the Saturated Hot Solution
We begin by dissolving potassium nitrate in hot water. We add so much of the solid that no more will dissolve, even with stirring. At this point, the solution is “saturated.” It contains the maximum possible amount of solute that the water can hold at that high temperature. The dissolved potassium nitrate particles (K⁺ and NO₃⁻ ions) are moving around rapidly, kept in solution by the energetic water molecules.
Step 2: The Cooling Process
As we allow this saturated solution to cool, the water loses thermal energy. This loss of energy is the critical trigger. The water’s capacity to hold potassium nitrate in solution diminishes. The solution, which was perfectly saturated at the high temperature, now becomes supersaturated as it cools.
A supersaturated solution is a temporary, unstable state. It contains more dissolved solute than it should theoretically be able to hold at the new, lower temperature. It’s like a crowded room that was just right when everyone was active, but now that everyone has settled down, the room feels uncomfortably packed.
Step 3: The Birth of a Crystal
In this crowded, supersaturated state, the dissolved ions start to seek a more stable, lower-energy arrangement. They begin to come together, forming tiny, ordered clusters. This process is called nucleation. A nucleation site can be a microscopic dust particle, a tiny imperfection on the glass surface, or even a single, chance grouping of ions that is stable enough to survive.
Step 4: Crystal Growth
Once a stable nucleus exists, it acts like a magnet. Other potassium and nitrate ions in the supersaturated solution are strongly attracted to this ordered structure. They preferentially latch onto the existing crystal, extending its pattern in a regular, repeating lattice. This is far more energetically favorable than remaining separate and dissolved in the now “overcrowded” water.
The system is essentially purging the excess solute it can no longer accommodate, organizing it into a highly ordered solid state—a crystal. This process continues, with ions continuously leaving the solution and joining the crystal, until the solution is no longer supersaturated. It reaches a new, stable saturation point for the final, cooler temperature.
A Simple Analogy: Dissolving and Re-forming Sugar
Think of making rock candy. You dissolve a massive amount of sugar in boiling water (creating a hot, saturated solution). As this syrup cools, it becomes supersaturated. When you dip a string into it, the sugar molecules slowly come out of the solution and build large, hard crystals on the string over several days. The cooling potassium nitrate solution does the same thing, just much faster.
Question 3.
Give three factors which affect the solubility of a solid solute in a solvent.
Ans:
1. Temperature
For the vast majority of solid solutes, solubility increases as the temperature of the solvent rises. This is because higher temperatures provide more kinetic energy to the solvent molecules and the solute particles. This enhanced energy helps to overcome the attractive forces holding the solid together, allowing more solute particles to be pulled into the solution and stabilized by the solvent. A common real-world example is how much more sugar can be dissolved in hot tea compared to iced tea.
2. The Nature of the Solute and Solvent (Polarity)
This is often summarized by the principle “like dissolves like.” A highly polar solute, such as table salt (ionic), will readily dissolve in a polar solvent like water, because the positive and negative ends of the water molecules can effectively surround and interact with the individual ions. Conversely, a non-polar solute, such as wax, will not dissolve in water but will dissolve in a non-polar solvent like hexane. The fundamental compatibility of the intermolecular forces (ionic, polar, or non-polar) between the solute and solvent is a primary determinant of whether a significant amount of dissolution will occur.
3. Particle Size of the Solute
While it does not change the maximum amount of solute that can dissolve (the equilibrium solubility), the physical size of the solute particles significantly affects the rate at which dissolution occurs. A finely powdered solute has a much larger total surface area exposed to the solvent compared to a single large crystal of the same mass. This greater surface area allows solvent molecules to contact and interact with more solute particles simultaneously, leading to a much faster dissolving process. For instance, fine granulated sugar dissolves far more quickly in coffee than a solid sugar cube.
Question 4.
1. If you are given some copper sulphate crystals, how would you proceed to prepare its saturated solution at room temperature?
2. How can you show that your solution is really saturated?
Ans:
1. Preparing a Saturated Solution of Copper Sulphate at Room Temperature
To prepare a saturated solution, you will need copper sulphate crystals, a clean beaker, distilled water, a glass rod, and a spatula. First, take about 100 ml of distilled water in the beaker. Begin adding a small amount of copper sulphate crystals to the water while stirring continuously with the glass rod. Keep adding and stirring until the crystals stop dissolving and you see some solid crystals settling at the bottom of the beaker, even after persistent stirring. At this point, the solution has reached its saturation point at that room temperature. To ensure it is perfectly saturated, allow the solution to sit undisturbed for a short while. The undissolved crystals at the bottom confirm that the solution can hold no more solute.
2. Demonstrating that the Solution is Saturated
Proving that the solution is truly saturated is a simple process. Once you have your prepared solution with excess crystals at the bottom, take a small, clean crystal of copper sulphate. Gently drop this crystal into the prepared solution without stirring. If the solution is saturated, you will observe that this new crystal does not dissolve. Instead, it will just settle at the bottom with the other undissolved crystals. This happens because the solution has already reached its maximum capacity for dissolving the solute at that specific temperature. The addition of a fresh crystal provides clear, visible proof that no more solute can be taken up by the solvent, confirming the saturated state of the solution.
Question 5.
(a) 1. Define Henry’s law
2. Define crystallisation.
3. Define Seeding
(b) State any three methods of crystallisation.
Ans:
(a) Definitions
Henry’s Law explains the relationship between gas and a liquid it’s dissolving into. It tells us that the amount of a gas that gets dissolved in a specific amount of liquid is directly tied to the pressure that same gas is applying on the liquid’s surface. You can think of a soda bottle; the high pressure inside forces the carbon dioxide gas to dissolve in the liquid, and when you open the cap, the pressure is released, and the gas fizzes out.
Crystallisation is a fantastic process used to get a pure solid material from its solution. It works by forcing the dissolved substance, or solute, to gracefully come out of the solution and arrange itself into structured, pure particles we know as crystals. This is usually achieved by carefully cooling a hot, saturated solution or by letting the solvent, like water, slowly evaporate away.
Seeding is a clever trick used to kick-start the crystallisation process when a solution is ready to form crystals but is hesitating. It involves dropping a tiny, pre-formed crystal of the same substance, called a seed crystal, into this supersaturated solution. This small crystal acts as a ready-made foundation or a template, encouraging the other dissolved particles to latch on and grow the crystal structure efficiently.
(b) Three Methods of Crystallisation
1. The Slow Evaporation Technique: This is the most straightforward approach. The solution is simply left in an open, shallow dish, allowing the solvent to vaporize into the air gradually at room temperature. As the liquid disappears, the solution becomes more and more crowded with the solute until it reaches a point where the solute molecules start grouping together to form crystals. This method works perfectly for substances like salt, whose solubility isn’t heavily affected by heat.
2. The Cooling Method: This technique is perfect for solids like copper sulphate or alum, which dissolve much more easily in hot water than in cold. Here, you first create a saturated solution by dissolving the maximum possible amount of the solute in hot solvent. This concentrated hot solution is then set aside to cool down slowly and peacefully. As the temperature falls, the solution’s capacity to hold the solute drops dramatically, compelling the excess solute to separate out in the form of beautiful, pure crystals.
3. The Non-Solvent Addition Method: Sometimes, the best way to make a substance form crystals is to change its environment. In this method, you add a second liquid, called a non-solvent, to the original solution. The key is that this new liquid must be one in which the solute does not dissolve at all, but it must be able to mix freely with the original solvent. When added, it “spoils” the solvent’s ability to keep the solute dissolved, drastically lowering its solubility and prompting it to crystallize out of the new mixture.
Question 6.
What would you observe when crystals of copper (II) sulphate and iron (II) sulphate are separately heated in two test tubes?
Ans:
Heating Copper(II) Sulphate Crystals (CuSO₄·5H₂O)
When the blue crystals of copper(II) sulphate are heated in a test tube, the following observations can be made:
- Colour Change: The most striking observation is the dramatic change in colour. The bright blue crystals gradually turn into a white or greyish-white powder.
- Condensation: Droplets of a colourless liquid (water) are seen condensing on the cooler upper walls of the test tube.
- Physical State: The solid crystals may appear to “crackle” slightly as they lose water and crumble into a powdery solid.
Explanation: The blue colour is due to water molecules that are chemically bound to the copper(II) sulphate, forming a hydrate (CuSO₄·5H₂O). Heating drives off this water of crystallisation, leaving behind anhydrous copper(II) sulphate (CuSO₄), which is white.
Heating Iron(II) Sulphate Crystals (FeSO₄·7H₂O)
Heating iron(II) sulphate crystals produces a more complex and dramatic set of observations:
- Initial Changes: The pale green crystals first lose their water of crystallisation. They often melt in their own water and appear as a colourless or pale solution bubbling inside the test tube.
- Colour Change: As heating continues, the colour of the substance changes dramatically. It turns from pale green to a dirty yellow or brownish solid.
- Gas Evolution: You will observe the evolution of gases. You may see steamy fumes (water vapour) and also smell the sharp, choking odour of sulphur dioxide (SO₂) gas.
- Condensation and Residue: Like with copper sulphate, water condenses on the cooler parts of the test tube. The final residue left in the test tube is a reddish-brown solid.
Explanation: Iron(II) sulphate decomposes upon strong heating; it does not simply dehydrate. The heat causes it to decompose into iron(III) oxide (which is reddish-brown), sulphur dioxide, and sulphur trioxide gases. This is an example of a decomposition reaction.
| Compound | Initial Appearance | Process and Chemical Change | Final Observation |
| Copper(II) Sulfate Pentahydrate (CuSO4⋅5H2O) | Bright Blue crystals | The heat causes the water of crystallization to evaporate. You would observe water droplets forming on the cooler, upper inner walls of the test tube. | The solid turns into a white or greyish-white powder (anhydrous copper(II) sulfate, CuSO4). |
| Iron(II) Sulfate Heptahydrate (FeSO4⋅7H2O) | Light Green crystals | Similar to copper sulfate, the seven molecules of water of crystallization evaporate due to the heat, which you would see condensing as water droplets. | The solid turns into a dirty white or off-white powder (anhydrous iron(II) sulfate, FeSO4). |
Question 7.
1. Give the names and formulae of two substances. Hydrated substance
2. Give the names and formulae of two substances. Anhydrous substance
3. Give the name and formula of two substances in the given case: Liquid drying agent 4. Give the names and formulae of two substances. A basic drying agent
Ans:
1. Two Hydrated Substances
Hydrated substances are chemical compounds that have water molecules attached to their crystal structure.
- Name: Copper(II) Sulfate Pentahydrate
Formula: CuSO₄·5H₂O
Commonly known as blue vitriol, it appears as bright blue crystals. - Name: Sodium Carbonate Decahydrate
Formula: Na₂CO₃·10H₂O
Known as washing soda, these are transparent, crystalline solid.
2. Two Anhydrous Substances
Anhydrous substances are compounds that contain no water molecules.
- Name: Anhydrous Calcium Chloride
Formula: CaCl₂
A white, powdery solid highly valued for its ability to absorb moisture from the air. - Name: Anhydrous Sodium Sulfate
Formula: Na₂SO₄
3. Two Liquid Drying Agents
These are substances in liquid form used to remove water from other compounds, typically by chemical reaction.
- Name: Concentrated Sulfuric Acid
Formula: H₂SO₄(l)
A highly effective and aggressive dehydrating agent used in industrial processes and desiccators. - Name: Phosphorus Pentoxide
Formula: P₄O₁₀
Note: While it is a solid at room temperature, it is so hygroscopic it often forms a viscous liquid layer on its surface as it reacts with atmospheric moisture, making it a common and powerful desiccant in a fluid state in chemical processes.
4. Two Basic Drying Agents
These are drying agents that are chemically basic (alkaline) in nature.
- Name: Calcium Oxide
Formula: CaO
Commonly known as quicklime, it reacts with water to form calcium hydroxide (slaked lime). - Name: Potassium Hydroxide
Formula: KOH
A strong base available in pellet form, it is very hygroscopic and effectively removes water vapor from gases.
Question 8.
What is the effect of temperature on solubility of KNO3 and CaSO4 in water?
Ans:
The solubility of a substance tells us how much of it can dissolve in a fixed amount of water to form a saturated solution. A key factor that influences this is temperature, but it doesn’t affect all substances in the same way. The general rule is that for most solid solutes, solubility increases as the temperature of the solvent rises. This happens because higher temperatures provide more kinetic energy, which helps break the solid’s crystal structure apart and allows its particles to mix more freely with the water molecules. However, this trend has important exceptions depending on the specific compound involved.
Taking potassium nitrate (KNO₃) as our first example, it is a classic case that perfectly follows the general rule. If you were to perform a simple lab experiment, you would observe that a very small amount of KNO₃ dissolves in a beaker of cold water. However, as you gently heat the water, you can see and measure that a significantly larger quantity of the same salt dissolves completely. This is why KNO₃ is often used to demonstrate the concept of recrystallization; when a hot, saturated solution is allowed to cool, the dissolved KNO₃ can no longer remain in solution and reforms into distinctive crystals.
In contrast, calcium sulfate (CaSO₄) behaves quite differently and represents an exception to the common trend. The solubility of CaSO₄ in water decreases slightly as the temperature increases. This counterintuitive behavior is linked to its exothermic dissolution process, meaning it releases heat when it dissolves. According to fundamental principles of chemistry, an exothermic process is less favored at higher temperatures. Therefore, for CaSO₄, warmer water provides a less hospitable environment for its dissolution, resulting in a lower solubility. This is a key reason why calcium sulfate can form hard, crusty deposits, known as scale, inside pipes and boilers that use hot water.
Question 9.
Solubility of NaCl at 40oC is 36.5 g. What is meant by this statement?
Ans:
This statement, “The solubility of NaCl at 40°C is 36.5 g,” is a precise scientific measurement that conveys a specific meaning about how much salt can dissolve in water under a defined set of conditions.
Here is a breakdown of what it means:
1. The Specific Components:
- Solute: The substance being dissolved, which is NaCl (sodium chloride, or common table salt).
- Solvent: The substance doing the dissolving. While not stated explicitly, it is universally understood to be water.
- Temperature: The specific condition of 40°C (which is 104°F).
2. The Core Meaning:
The number 36.5 g refers to the maximum mass of NaCl that can completely dissolve in 100 grams of pure water at the specified temperature of 40°C to form a stable, saturated solution.
In simpler terms:
If you were to take 100 grams of water and gradually add NaCl to it at 40°C, you would find that no more than 36.5 grams of the salt would dissolve and disappear into the water. Any additional salt you add after this point would simply sink to the bottom and remain as undissolved solid, no matter how much you stir.
3. Key Implications of this Statement:
- It Defines a Saturated Solution: When exactly 36.5 g of NaCl is dissolved in 100 g of water at 40°C, the solution is “saturated.” It has reached its maximum capacity to hold the solute.
- It is a Point of Equilibrium: In a saturated solution, a dynamic equilibrium exists. Salt crystals are simultaneously dissolving at the same rate as dissolved salt ions are re-crystallizing. The net amount of dissolved salt remains constant.
- Temperature is Crucial: This value is only true for 40°C. At a lower temperature (like 20°C), less salt would dissolve. At a higher temperature, a slightly different amount would dissolve (though the solubility of NaCl changes very little with temperature compared to other salts).
Question 10.
Which test will you carry out to find out if a given solution is saturated or unsaturated or supersaturated?
Ans:
To determine the nature of a solution, a simple and effective test is the seed crystal test. This method relies on the fundamental behavior of solutes in a solution at a given temperature.
First, you would take a small, pure crystal of the same solute that is dissolved in the solution you are testing. For example, if the solution is saltwater, you would use a tiny grain of salt. Gently drop this seed crystal into the solution and observe what happens carefully. If the crystal sinks to the bottom without any change in size, the solution is unsaturated. This is because an unsaturated solution has the capacity to dissolve more solute, so the added crystal simply dissolves away.
If, however, the seed crystal triggers a process where additional solute begins to crystallize out of the solution and deposit onto your seed (making it grow), or if new crystals start forming throughout the solution, you have identified a supersaturated solution. A supersaturated solution is unstable and holds more solute than it normally should; the seed crystal provides a surface for this excess solute to come out of the solution. If you see no dissolution but also no new crystal growth, the solution is in a state of equilibrium, meaning it is saturated. It cannot dissolve any more solute, but it is also not holding an excess amount, so the seed crystal remains unchanged.
Question 11.
What is the effect of pressure on solubility of gases? Explain with an example.
Ans:
How Pressure Influences Gas Solubility in Liquids
The amount of a gas that can dissolve in a liquid is heavily dependent on the pressure of that gas above the liquid’s surface. A simple way to understand this is: push the gas down, and more of it will be absorbed by the liquid. Relieve that pressure, and the dissolved gas will energetically make its exit.
This fundamental concept is formally described by Henry’s Law.
The Molecular “Dance” at the Surface
To grasp why this happens, picture the boundary between a liquid and the gas above it. A dynamic exchange is constantly underway. Molecules of the gas are continually colliding with the liquid’s surface, and a portion of these molecules becomes trapped, or dissolved, within the liquid. Simultaneously, dissolved gas molecules inside the liquid are moving with enough energy to break free and re-enter the gas phase.
A balance is achieved when the number of gas molecules entering the liquid in a given time equals the number escaping from it. This is a state of equilibrium.
Now, consider what occurs when the pressure is increased. This action forces a greater number of gas molecules into the space above the liquid. Consequently, the “rain” of gas molecules striking the liquid surface becomes more intense. With more frequent impacts, a larger number of molecules successfully penetrate the liquid. The system is temporarily thrown off balance. To restore equilibrium, the rate of dissolution increases until it once again matches the rate of escape, but now at a higher concentration of dissolved gas.
A Practical Illustration: The Fizz in Your Soda
The entire lifecycle of a carbonated beverage is a perfect demonstration of this principle in action.
- Creation Under Pressure:
During production, carbon dioxide (CO₂) is forced into the drink under significantly elevated pressure. This high-pressure environment coaxes an unusually large amount of CO₂ to dissolve into the liquid, creating a supersaturated solution. The container is then sealed, locking in this high-pressure state. - Stability in a Sealed Container:
Inside the closed bottle or can, an equilibrium exists. The high concentration of dissolved CO₂ in the liquid is balanced by the high pressure of the CO₂ gas in the headspace. The system is stable, and the gas remains in solution. - The Moment of Release:
The pivotal moment occurs when you open the container. The distinctive “hiss” is the sound of high-pressure gas rushing out. Instantly, the pressure above the liquid plummets to normal atmospheric levels. According to the gas law, the liquid can no longer sustain its high concentration of dissolved gas under these new, low-pressure conditions.
The dissolved CO₂, now in excess, must escape. It rapidly forms tiny bubbles at nucleation sites (like imperfections in the glass or specks of dust) and rises to the surface in the effervescent fizz we all recognize. If the bottle is left uncapped, this process will continue until the drink reaches a new, much lower equilibrium with the air, resulting in a flat beverage.
Question 12.
1. State the term: (Do not give examples) A solution where solvent is a liquid other than water.
2. State the term: When a substance absorbs moisture on exposure to moist air and dissolves in the absorbed water and turns to solution.
3. State the term: A substance which contains water of crystallisation.
4. When a substance absorbs moisture from the atmosphere but does not form a solution.
5. When a compound loses its water of crystallisation on exposure to dry air.
6. The substance that can remove hydrogen and oxygen atoms in the ratio of 2:1(in the form of water) from the compound.
Ans:
- Non-aqueous solution
- Deliquescence
- Hydrated salt
- Hygroscopy
- Efflorescence
- Dehydrating agent
Question 13.
1. Explain why: Water is an excellent liquid to use in cooling systems.
2. Explain why: A solution is always clear and transparent.
3. Explain why: Lakes and rivers do not suddenly freeze in the winters.
4. Explain why: The solute cannot be separated from a solution by filtration.
5. Explain why: Fused CaCl2 or conc. H2SO4 is used in a desiccator.
6. Explain why: Effervescence is seen on opening a bottle of soda water.
7. Explain why: Table salts become sticky on exposure to humid air during the rainy season.
Ans:
1. Why Water is an Excellent Liquid to Use in Cooling Systems
Water is an ideal choice for cooling systems due to a unique combination of physical properties. Its most crucial attribute is its exceptionally high specific heat capacity. This means water can absorb a large amount of heat energy from an engine or industrial process while its own temperature rises only slightly. This allows a relatively small volume of water to effectively carry away a significant amount of heat. Furthermore, water has high thermal conductivity, enabling it to rapidly distribute the absorbed heat throughout its volume, promoting efficient heat exchange in the radiator. It is also readily available, non-toxic, and inexpensive, making it a practical and efficient coolant.
2. Why a Solution is Always Clear and Transparent
A solution is a homogeneous mixture where the solute particles (the substance being dissolved) are broken down to the level of individual atoms, ions, or molecules. These particles are so incredibly small (typically less than 1 nanometer in diameter) that they cannot scatter beams of light passing through the mixture. Since the light passes through without being obstructed or scattered, the solution appears clear and transparent to our eyes. This distinguishes a true solution from a suspension or colloid, where larger particles can scatter light, making them appear cloudy or opaque.
3. Why Lakes and Rivers Do Not Suddenly Freeze in the Winters
This phenomenon is a direct result of water’s unusual property: it is densest at 4°C. As the surface water cools, it becomes denser and sinks, pushing the warmer water upward in a process called convection. This circulation continues until the entire body of water reaches 4°C. Only then does the surface water cool further to 0°C and begin to freeze. Because ice is less dense than liquid water, it forms a floating insulating layer on top. This layer of ice slows down the further loss of heat from the water below, preventing the lake or river from freezing solid all at once.
4. Why The Solute Cannot Be Separated from a Solution by Filtration
In a true solution, the solute particles are dissolved at the molecular or ionic level. They are far too small to be trapped by the pores of a filter paper. Filtration works by physically separating larger, undissolved solid particles from a liquid or gas. Since the solute particles in a solution are essentially part of the liquid itself, they simply pass through the filter along with the solvent. To separate a solute from a solution, processes that rely on a change of state are required, such as evaporation or distillation.
5. Why Fused CaCl₂ or Conc. H₂SO₄ is Used in a Desiccator
A desiccator is a sealed container used to keep substances dry by removing moisture from the air inside it. Fused (anhydrous) Calcium Chloride (CaCl₂) and concentrated Sulfuric Acid (H₂SO₄) are powerful desiccating agents, or desiccants. They are highly hygroscopic, meaning they have a very strong chemical affinity for water vapor and will readily absorb it from the surrounding atmosphere. By placing a dish of these substances inside the desiccator, they actively and continuously dry the air, creating a moisture-free environment to protect samples from humidity.
6. Why Effervescence is Seen on Opening a Bottle of Soda Water
Soda water is manufactured by dissolving carbon dioxide (CO₂) gas in water under high pressure. When the bottle is sealed, the high pressure inside forces the CO₂ to stay dissolved in the liquid. Upon opening the bottle, the pressure is suddenly released. According to gas laws, a gas becomes less soluble in a liquid as the pressure decreases. The dissolved CO₂ can no longer be held in the solution and rapidly escapes in the form of bubbles. This rapid release of gas bubbles is what we observe as effervescence or fizzing.
7. Why Table Salt Becomes Sticky on Exposure to Humid Air During the Rainy Season
Common table salt (NaCl) often contains small amounts of impurities like Magnesium Chloride (MgCl₂), which is highly deliquescent. Deliquescent substances have such a strong tendency to absorb moisture from the air that they eventually dissolve in the absorbed water and form a liquid solution. During the rainy season, the relative humidity is very high, meaning the air is laden with moisture. The impurities in the salt absorb this moisture, forming a thin, saturated brine solution on the surface of the salt crystals. This layer of sticky liquid causes the individual salt crystals to clump together and become damp and sticky.
Question 14.
1. Normally, solubility of crystalline solid increases with temperature. Does it increase uniformly in all cases? Name a substance whose solubility: Increases rapidly with temperature
2. Normally, solubility of crystalline solid increases with temperature. Does it increase uniformly in all cases? Name a substance whose solubility: Increases gradually with temperature.
3. Normally, solubility of crystalline solid increases with temperature. Does it increase uniformly in all cases? Name a substance whose solubility: Increases slightly with temperature.
4. Normally, solubility of crystalline solid increases with temperature. Does it increase uniformly in all cases? Name a substance whose solubility: Initially increases then decreases with rise in temperature.
Ans:
1. No, the increase in solubility with temperature is not uniform for all crystalline solids. The degree of increase varies greatly from one substance to another based on its internal structure and how it interacts with water molecules. A classic example of a substance whose solubility increases rapidly with temperature is potassium nitrate (KNO₃). If you look at a solubility curve, the line for potassium nitrate is very steep, showing that a small rise in temperature allows a much larger amount of it to dissolve in water.
2. The relationship between temperature and solubility is indeed diverse and not a one-size-fits-all rule. Many substances show a more moderate response to heat. A common substance whose solubility increases gradually with temperature is potassium chloride (KCl). Its solubility curve is a steadily rising line, indicating a consistent but gentle increase in the amount that can dissolve as the water gets warmer.
3. For some solids, the dissolving process is not heavily dependent on thermal energy. In these cases, the solubility changes very little. A well-known example of a substance whose solubility only increases slightly with temperature is sodium chloride (NaCl), or common table salt. Its solubility curve is almost a flat line, demonstrating that you can only dissolve a marginally greater amount of salt in very hot water compared to cold water.
4. There are even more exceptional cases that defy the general trend. A few substances exhibit a unique solubility pattern where it initially increases then decreases with a rise in temperature. The most frequently cited example of this behavior is sodium sulfate decahydrate (Na₂SO₄·10H₂O), also known as Glauber’s salt. Its solubility increases up to a certain temperature (around 32.4°C) and then begins to decrease as the temperature climbs further.
Question 15.
What are drying or desiccating agents? Give examples.
Ans:
Drying or desiccating agents are substances that have a very strong ability to absorb moisture from the air or other gases, and in some cases, from liquids. They work either by physically absorbing water, like a sponge, or by chemically reacting with it to form a new compound. The key feature of a good desiccating agent is its hygroscopic nature, meaning it pulls and holds water vapor from its surroundings, thereby creating a dry environment. This process is called desiccation.
Common examples of these agents are used frequently in everyday life and laboratories. For instance, the small silica gel packets found in new shoe boxes or electronics packaging are a very common physical drying agent; the silica gel beads absorb moisture to prevent damage from dampness. In chemical laboratories, concentrated sulphuric acid is a classic example of a powerful desiccant that removes water through a chemical reaction. Other strong examples include calcium oxide (quicklime) and calcium chloride, which are often used to dry gases because of their high affinity for water.
Question 16.
Complete the following table:
| Common Name | Chemical Name | Formula | Acid, base or salt | Efflorescent,hygroscopic or deliquescent substance |
| Solid caustic potash | ||||
| Quick lime | ||||
| Oil of vitriol | ||||
| Washing soda | ||||
| Solid caustic soda | ||||
| Blue vitriol |
Ans:
| Common Name | Chemical Name | Formula | Acid, Base, or Salt | Moisture Behavior |
| Solid caustic potash | Potassium Hydroxide | KOH | Base (Strong Alkali) | Deliquescent (Absorbs enough moisture to dissolve and form a solution) |
| Quick lime | Calcium Oxide | CaO | Base (Basic Oxide) | Hygroscopic (Absorbs moisture from the air) |
| Oil of vitriol | Sulphuric Acid | H2SO4 | Acid (Strong Acid) | Hygroscopic (Strong dehydrating agent, readily absorbs water vapor) |
| Washing soda | Sodium Carbonate Decahydrate | Na2CO3⋅10H2O | Salt (of a strong base and weak acid) | Efflorescent (Loses water of crystallization to the air) |
| Solid caustic soda | Sodium Hydroxide | NaOH | Base (Strong Alkali) | Deliquescent (Absorbs enough moisture to dissolve and form a solution) |
| Blue vitriol | Copper(II) Sulphate Pentahydrate | CuSO4⋅5H2O | Salt (of a strong acid and weak base) | Efflorescent (Loses water of crystallization to the air upon drying/heating) |
Question 17.
1. In which of the following substances will there be Decrease in mass
2. In which of the following substances will there be Increase in mass
3. In which of the following substances will there be no change in mass when they are exposed to air?
Sodium chloride
Iron
Conc. sulphuric acid
Table salt
Sodium carbonate crystals
Ans:
1. In which of the following substances will there be a decrease in mass?
Conc. Sulphuric Acid
- Reason: Concentrated sulphuric acid (H₂SO₄) is highly hygroscopic, meaning it has a strong affinity for water vapor in the air. However, in this specific case, it does not just absorb water; it acts as a dehydrating agent. When exposed to air, it will vigorously absorb moisture and lose its own molecules as a mist or fume. This results in a gradual loss of mass over time as the acid’s composition changes and it becomes diluted, with some components escaping into the atmosphere.
2. In which of the following substances will there be an increase in mass?
Iron, Sodium Carbonate Crystals, and (to a lesser extent) Table Salt
- Iron: Iron (Fe) undergoes a chemical reaction with oxygen and moisture in the air, a process known as rusting. This forms hydrated iron(III) oxide (Fe₂O₃·xH₂O). Since oxygen atoms from the air and water molecules become chemically bonded to the iron, the total mass of the substance increases significantly.
- Sodium Carbonate Crystals (Washing Soda – Na₂CO₃·10H₂O): This substance exists as crystals that contain water molecules within their structure (water of crystallization). When exposed to dry air, it doesn’t lose water; instead, it can absorb more moisture from humid air. This process, called efflorescence, is often misunderstood. While the crystals may crumble as they lose their structure, the primary interaction with moist air leads to them gaining additional water molecules, increasing their mass before they eventually dissolve into a solution or lose their form.
- Table Salt (if impure): Pure, dry sodium chloride is very stable. However, common table salt often contains impurities like magnesium chloride (MgCl₂), which is hygroscopic. These impurities cause the salt to absorb moisture from the air and form a damp cake, leading to an increase in mass.
3. In which of the following substances will there be No Change in mass when they are exposed to air?
Sodium Chloride (Pure)
- Reason: Pure sodium chloride (NaCl) is a stable, non-hygroscopic compound. It does not react with any of the major components of air (nitrogen, oxygen, carbon dioxide) under normal conditions, and it does not absorb significant amounts of water vapor. Therefore, its mass remains constant when left exposed to air.
Question 18.
State the methods by which hydrated salts can be made anhydrous.
Ans:
One common method to drive off water of crystallization is through heating. When a hydrated salt is warmed strongly, the water molecules, which are loosely bound within the crystal structure, gain enough energy to break free as vapor. A classic example you might have seen in a lab is heating blue hydrated copper sulphate crystals, which steadily lose their water content and turn into a white, powdery anhydrous form. This process effectively removes the water molecules that are physically trapped in the crystal lattice.
Another technique, suitable for salts that might decompose upon heating, involves using a desiccant. The hydrated salt is placed in a desiccator, which is a sealed container containing a powerful drying agent like concentrated sulphuric acid or silica gel. These desiccants have a very strong affinity for water vapor and gradually absorb the moisture from the hydrated salt from the surrounding air, leaving behind the anhydrous solid. This method is much gentler than direct heating and is essential for processing heat-sensitive compounds.
Exercise 3 (C)
Question 1.
What is the composition of water? In what volume its elements combine?
Ans:
The composition of water is not a mixture but a definite chemical compound. Its molecule is formed by the chemical combination of two fundamental elements: hydrogen and oxygen. Specifically, every single molecule of water (H₂O) consists of two atoms of hydrogen chemically bonded to one atom of oxygen. This specific and unchanging ratio is what defines water as a pure substance.
When it comes to the volume in which these gaseous elements combine, the relationship is a fascinating and fixed one. According to Avogadro’s law, gases combine in simple whole-number ratios by volume. In the case of water, the two constituent elements, hydrogen and oxygen gas, combine in a fixed volume ratio of 2:1. This means that to form water vapor, two volumes of hydrogen will always react with exactly one volume of oxygen.
Question 2.
What is the use of solubility of oxygen and carbon dioxide in water?
Ans:
The presence of oxygen and carbon dioxide dissolved in water is a simple natural process with powerful, real-world effects.
Dissolved Oxygen: The Breath of Water
Dissolved oxygen is essential for the health of rivers, lakes, and oceans. It is the lifeblood for fish and other aquatic creatures, allowing them to breathe. It also supports the beneficial bacteria that clean the water by breaking down waste.
This makes oxygen levels a direct measure of water quality. A sudden drop often signals pollution, as excess nutrients can trigger algal blooms that decay and consume all the oxygen, creating “dead zones.” To prevent this, wastewater treatment plants and fish farms use aerators to pump oxygen into the water, ensuring survival and cleanliness.
Dissolved Carbon Dioxide: A Multitasking Molecule
Carbon dioxide plays several critical roles. On a global scale, the oceans absorb vast amounts of CO₂ from the air, acting as a crucial buffer against climate change. However, this also causes ocean acidification, which harms shell-building animals like corals.
In our daily lives, dissolved CO₂ is what creates the fizz in sodas and sparkling waters. The same mild acidity that gives these drinks their tang also acts as a natural preservative. Furthermore, this gas is used in specialized firefighting systems, where carbonated water creates a smothering fog to put out flames.
A Delicate Partnership
These two gases exist in a vital balance. Underwater plants use dissolved CO₂ for photosynthesis and release the oxygen that animals need to survive. This continuous exchange, made possible by their ability to dissolve in water, sustains aquatic ecosystems and supports a wide range of human needs.
Question 3.
A hot saturated solution of sodium nitrate forms crystals as it cools. Why?
Ans:
The solubility of a solid in a liquid, like sodium nitrate in water, is highly dependent on temperature. Generally, for most solids, hot water can hold a much greater amount of the dissolved substance compared to cold water. A saturated solution is one that has reached its maximum capacity for dissolving the solute at a specific temperature; it simply cannot hold any more.
When you have a hot saturated solution of sodium nitrate, it means the water is holding the absolute maximum amount of sodium nitrate it possibly can at that high temperature. As this solution is allowed to cool down, the water’s capacity to hold the dissolved sodium nitrate decreases. The water molecules have less energy and cannot keep as much of the solid particles separated and in solution.
Because the solution was already saturated at the higher temperature, this decrease in solubility forces the excess sodium nitrate to leave the solution. This excess solute does not simply sink to the bottom as a powder; instead, it comes out of the solution in a very organized, structured form, which we observe as crystals. In essence, cooling the solution makes it supersaturated for a moment, and the formation of crystals is the system’s way of returning to a stable, saturated state at the new, lower temperature.
Question 4.
What are hydrous substances? Explain with examples.
Ans:
Hydrous Substances
Hydrous substances are chemical compounds that have water molecules integrated as a fundamental part of their crystalline structure. This isn’t water that is just physically mixed or trapped; it is chemically bound within the crystal lattice in a fixed proportion. The water molecules in these substances are known as water of crystallization, and they are responsible for giving the compound its specific crystalline shape, color, and sometimes its stability. When you heat a hydrous substance, it loses this water and often undergoes a visible change, such as turning from a colored crystal to a white powder.
A classic and simple example is Copper Sulphate. Its chemical formula is CuSO₄·5H₂O, which tells us that for every one unit of copper sulphate, there are five molecules of water of crystallization. In its hydrous form, it exists as beautiful blue crystals. However, when these blue crystals are heated strongly, they lose their water molecules and transform into a white, powdery substance known as anhydrous copper sulphate (CuSO₄). This change is reversible, as adding water to the white powder will restore its blue color.
Another common example is Washing Soda, or Sodium Carbonate Decahydrate (Na₂CO₃·10H₂O). This compound contains ten water molecules for every sodium carbonate unit. These water molecules are crucial for its large, colorless crystals and its use as a cleaning agent. Similarly, Gypsum, a mineral used to make Plaster of Paris, is a hydrous calcium sulphate with the formula CaSO₄·2H₂O. When heated, it loses some of its water to form the hemihydrate, which is the basis for the plaster used in construction and casts. In essence, the ‘hydrous’ nature defines the very identity and properties of these common substances.
Question 5.
Name three methods by which hydrous substances can be made anhydrous.
Ans:
- Thermal Dehydration:
This method uses controlled heat to break the bonds between the water molecules and the host substance. The energy provided by the heat overcomes the forces (like hydrogen bonding or coordination) holding the water in place, causing it to vaporize and escape. A common laboratory example is gently heating hydrated copper(II) sulfate, which is blue, to drive off the water and produce white, anhydrous copper(II) sulfate powder. - Chemical Desiccation:
This technique employs a highly reactive chemical agent, known as a desiccant, to sequester water molecules. The desiccant undergoes an irreversible chemical reaction with water, effectively removing it from the surrounding environment or a substance dissolved in a solvent. For instance, reactive metals like sodium metal are used to remove trace water from organic solvents like ethanol because the sodium reacts violently with water to form sodium hydroxide and hydrogen gas, leaving the dry ethanol behind. - Azeotropic Distillation:
This is a more specialized method used primarily for liquids. A water-immiscible solvent, such as toluene or benzene, is added to the hydrous substance. When this mixture is boiled, the solvent and water form a low-boiling “azeotrope” that vaporizes together. This vapor is then condensed and collected, and because the solvent and water are immiscible, they separate into two layers in the receiving vessel. The water layer can be physically separated, continuously removing water from the original substance until it is anhydrous.
Question 6.
What is the importance of dissolved impurities in water?
Ans:
On one hand, many dissolved impurities are absolutely essential for life. The most crucial are the mineral salts, like salts of calcium, magnesium, potassium, and sodium, which are dissolved as water flows over and through rocks and soil. For aquatic plants, these minerals are their primary source of nutrients, absorbed directly from the water for growth and development. For terrestrial animals and humans, the water we drink serves as a major dietary source of these essential electrolytes. They are vital for nerve function, muscle contraction, and maintaining proper fluid balance within our bodies. In fact, completely pure, distilled water is not ideal for regular consumption as it can leach these very minerals from the body. Furthermore, dissolved oxygen and carbon dioxide are gaseous impurities fundamental to survival, enabling aquatic respiration and photosynthesis.
Conversely, the presence of certain dissolved impurities poses serious threats. Harmful chemicals like heavy metals (lead, mercury, arsenic), excessive nitrates from fertilizers, and industrial pollutants can contaminate water sources. These toxic substances can accumulate in the bodies of aquatic organisms, poisoning them and disrupting entire food webs, eventually reaching humans. High concentrations of dissolved salts, as seen in seawater or brackish water, make it unfit for drinking or irrigation as it dehydrates cells. For industrial purposes, dissolved salts like calcium bicarbonate cause “hardness” in water. This leads to scaling inside boilers and pipes, reducing their efficiency and lifespan, and interferes with the lathering of soaps, making cleaning difficult. Therefore, while some dissolved impurities are the very essence of life, others can be agents of poisoning, corrosion, and inefficiency.
Question 7.
State two ways by which a saturated solution can be changed to an unsaturated solution.
Ans:
- Introduce More Liquid (Solvent): By adding more of the liquid (the solvent) to the saturated solution, you are essentially spreading the dissolved particles out more thinly. This increases the solution’s capacity to hold more of the solid, thereby changing its status from saturated (full) to unsaturated (can hold more).
- Apply Heat to the Solution: For most solid solutes, raising the temperature of a saturated solution allows the liquid to dissolve more of the solid. The added energy breaks apart the solid’s structure more effectively, creating an unsaturated condition where additional solute can then be introduced and dissolved.
Question 8.
1. What do you understand by Soft water
2. What do you understand by Hard water
3. What do you understand by Temporary hard water
4. What do you understand by Permanent hard water
Ans:
1. Soft Water
Soft water is water that readily forms a lather with soap because it lacks or has very low concentrations of dissolved calcium and magnesium salts. These specific minerals are the main culprits that prevent soap from lathering easily. Since it doesn’t contain these interfering minerals, soap can dissolve and foam effectively, making it very efficient for washing clothes, bathing, and other cleaning tasks. It also helps prevent the scale buildup in pipes, kettles, and industrial boilers that can reduce their efficiency and lifespan.
2. Hard Water
Hard water is the opposite of soft water. It is defined by its inability to easily form a lather with soap due to the presence of high concentrations of dissolved minerals, primarily calcium and magnesium ions. When soap is added to hard water, it first reacts with these minerals to form an insoluble, white precipitate often seen as scum. Only after all the dissolved minerals have reacted with the soap will a lather begin to form, meaning more soap is required for cleaning. This property is the most common way to identify hard water in a simple test.
3. Temporary Hard Water
Temporary hard water is a specific type of hard water whose hardness can be removed by simply boiling the water. This hardness is caused by the presence of dissolved calcium bicarbonate or magnesium bicarbonate. When the water is heated, these bicarbonates decompose. This reaction forms insoluble carbonates, which appear as the white, chalky solid known as scale at the bottom of a kettle, and carbon dioxide gas. Once these bicarbonates are removed through boiling, the water loses its hardness and becomes soft.
4. Permanent Hard Water
Permanent hard water is another type of hard water whose hardness cannot be eliminated by boiling. This is because it is caused by dissolved salts other than bicarbonates, typically calcium sulfate or magnesium sulfate (or chlorides). These salts are stable and do not break down when heated. Therefore, boiling does not remove them from the solution, and the water remains hard. To soften permanent hard water, more advanced chemical methods are required, such as adding washing soda or using ion-exchange processes.
Question 9.
1. What are the causes for: Temporary hardness
2. What are the causes for: Permanent hardness
Ans:
1. Causes of Temporary Hardness
Temporary hardness gets its name because it can be easily removed by simple boiling. The primary cause is the presence of specific dissolved minerals.
- The Main Culprit: Calcium Hydrogencarbonate
The chief cause of temporary hardness is the high concentration of calcium hydrogencarbonate [Ca(HCO₃)₂] dissolved in the water. Magnesium hydrogencarbonate [Mg(HCO₃)₂] can also contribute, but to a lesser extent. - How It Forms: A Natural Chemical Reaction
This doesn’t come from minerals dissolving directly. Instead, it forms through a fascinating two-step natural process:- Rainwater Reacts with Air: As rainwater falls through the atmosphere, it absorbs carbon dioxide (CO₂), forming a weak acid known as carbonic acid (H₂CO₃).
CO₂ + H₂O → H₂CO₃ - The Acid Attacks Rock: This slightly acidic water then percolates through the ground over limestone or chalk bedrock (which is primarily calcium carbonate, CaCO₃). The carbonic acid reacts with the otherwise insoluble calcium carbonate, converting it into the highly soluble calcium hydrogencarbonate.
CaCO₃ + H₂CO₃ → Ca(HCO₃)₂
- Rainwater Reacts with Air: As rainwater falls through the atmosphere, it absorbs carbon dioxide (CO₂), forming a weak acid known as carbonic acid (H₂CO₃).
- This process is why water from underground aquifers or springs in limestone-rich regions is often very hard. The water has effectively “captured” the hardness in a soluble form after a chemical interaction between the rock and the acidic rain.
2. Causes of Permanent Hardness
Permanent hardness is more stubborn and, as the name implies, is not removed by boiling. It is caused by a different set of dissolved salts.
- The Main Culprits: Sulfates and Chlorides
The primary drivers of permanent hardness are the calcium and magnesium salts of strong acids. The most common ones are:- Calcium Sulfate (CaSO₄)
- Magnesium Sulfate (MgSO₄)
- Calcium Chloride (CaCl₂)
- Magnesium Chloride (MgCl₂)
- How It Forms: Direct Dissolution and Other Reactions
Unlike temporary hardness, these compounds can dissolve directly into the water from the surrounding geology without needing a complex reaction with carbonic acid.- Direct Dissolution from Gypsum: A major source is the mineral gypsum (calcium sulfate dihydrate, CaSO₄·2H₂O). This mineral is relatively soluble in water, so groundwater flowing over gypsum-rich deposits will directly dissolve these salts, introducing permanent hardness.
- Ion Exchange in Soil: In some soils, calcium and magnesium ions can be released and combined with sulfate or chloride ions that are already present from other mineral sources or from agricultural runoff.
- Boiling Doesn’t Work: When you boil water with permanent hardness, these sulfates and chlorides do not precipitate out as solid scale. They remain in their dissolved ionic form in the water because their solubility is not significantly reduced by an increase in temperature, unlike the hydrogen carbonates.
Question 10.
1. What are the advantages of soft water?
2. What are the advantages of hard water?
Ans:
1. Advantages of Soft Water
The primary benefit of soft water is its superior performance in cleaning tasks. Because it contains minimal levels of calcium and magnesium ions, it allows soap and detergent to lather effortlessly. This means you use significantly less shampoo, body wash, laundry detergent, and dish soap to achieve a rich, effective lather. For your clothes, this results in brighter colors and softer fabrics, as no soap scum is left trapped in the fibers. In the home, soft water prevents the unsightly and damaging scale buildup in pipes, water heaters, kettles, and showerheads. This not only extends the lifespan of your appliances but also improves their energy efficiency, as a scaled-up water heater doesn’t have to work as hard to heat the water. For personal care, soft water leaves skin feeling less dry and hair more manageable, as it rinses clean without any mineral residue.
2. Advantages of Hard Water
While often seen as a nuisance for cleaning, hard water offers some notable, often overlooked health and dietary benefits. The very minerals that cause scale—calcium and magnesium—are essential nutrients for the human body. Regular consumption of hard water can contribute a small but valuable amount of these minerals to your daily dietary intake, which is beneficial for maintaining strong bones and proper nerve and muscle function. Furthermore, the taste of water is often perceived as better and more substantial when it contains these dissolved minerals, compared to the sometimes flat taste of soft water. From a health perspective, there is some evidence to suggest that the minerals in hard water can have a protective effect on cardiovascular health. The scale formation inside pipes, while problematic, can also form a protective coating that reduces the corrosion of the pipes from the water itself, potentially limiting the leaching of metals like lead or copper from older plumbing systems into the water supply.
Question 11.
What are stalagmites and stalactites? How are they formed?
Ans:
The Silent Growth of Caves: Stalactites and Stalagmites
Hidden in the world’s deep caves, a grand, imperceptibly slow artwork is continuously shaped. The primary sculptors are water and mineral, working over spans of time that dwarf human history. Their creations are the stone icicles and pillars known as stalactites and stalagmites. Though frequently named together, they are different formations, and a simple trick helps distinguish them.
Stalactites extend from the cave’s ceiling.
Stalagmites rise from the cave’s floor.
The Origin of Cave Sculptures
This remarkable process starts not underground, but at the surface. Rainwater, which is naturally mildly acidic, absorbs carbon dioxide from the atmosphere and from soil. This reaction creates a weak solution called carbonic acid.
This acidic water then filters down through the ground, passing layers of rock. When it moves through limestone, which is composed of calcium carbonate, it slowly dissolves the rock. The water, now carrying this dissolved mineral in solution, eventually finds its way into a cave through a fissure or pore in the ceiling.
Inside the cave, the environment triggers a change. The air here has a different concentration of carbon dioxide compared to the soil above. This shift causes the mineral-laden water droplet to release its dissolved carbon dioxide, much like the fizz leaving an open soda bottle. As the gas escapes, the water can no longer keep the limestone dissolved. The mineral begins a transformation from a liquid solution back into a solid state.
The formation unfolds in a repetitive cycle:
- A Drop Gathers: A bead of water, saturated with dissolved calcite (a crystal form of calcium carbonate), emerges from a crack and clings to the ceiling.
- A Deposit Remains: While the droplet hangs, a small amount of evaporation occurs. Crucially, the loss of carbon dioxide forces the water to deposit a microscopic ring of calcite around the point of contact on the rock.
- The Structure Grows: The water droplet falls, but the tiny mineral ring remains. The next droplet follows the same path, adding another infinitesimal layer. Over millennia, these accumulated deposits grow downward, first forming a delicate, hollow tube known as a “soda straw” stalactite. If the water flow increases and begins to run along the outside of this tube, more calcite is laid down, creating the classic, cone-shaped stalactite.
Question 12.
1. Name the substance which makes water temporarily hard.
2. Name the substance which makes water permanently hard.
Ans:
1. The temporary hardness of water is primarily caused by the presence of dissolved calcium hydrogencarbonate. This particular compound forms when water containing carbon dioxide, essentially a weak acid, filters through limestone deposits (calcium carbonate). This reaction dissolves the otherwise insoluble limestone, allowing the calcium to enter the water supply. The “temporary” part of its name comes from its behavior when heated. Boiling the water drives off the carbon dioxide, causing the calcium hydrogencarbonate to revert back to its original, insoluble form. This is why we see limescale, which is primarily calcium carbonate, building up inside kettles and boilers.
2. On the other hand, permanent hardness is introduced into water by salts like calcium sulphate. These minerals dissolve as water moves over gypsum-containing rocks and soils. The key difference here is that these salts are stable and do not break down or precipitate out of the water when it is boiled. Because heating has no effect, removing this type of hardness requires a different approach, specifically chemical precipitation. Common methods include adding washing soda (sodium carbonate) or using ion-exchange resins, which trap the calcium and magnesium ions responsible for the hardness and release sodium ions in their place, effectively softening the water.
Question 13.
1. Give equations to show what happens when temporary hard water is Boiled
2. Give equations to show what happens when temporary hard water is Treated with slaked lime
Ans:
1. When Temporary Hard Water is Boiled
Boiling temporary hard water provides heat energy, which causes the dissolved calcium hydrogencarbonate to decompose. This reaction forms insoluble calcium carbonate, which is the white precipitate you see as “limescale,” along with water and carbon dioxide gas.
Chemical Equation:
Ca(HCO₃)₂(aq) → CaCO₃(s) + H₂O(l) + CO₂(g)
In Words:
Aqueous Calcium Hydrogencarbonate decomposes to form a solid precipitate of Calcium Carbonate, liquid Water, and Carbon Dioxide gas.
2. When Temporary Hard Water is Treated with Slaked Lime
Slaked lime (Calcium Hydroxide) is added to react with the dissolved calcium hydrogencarbonate. The hydroxide ions from the lime react with the hydrogencarbonate ions to form carbonate ions. These carbonate ions then combine with calcium ions to form the insoluble calcium carbonate, which precipitates out.
Chemical Equation:
Ca(HCO₃)₂(aq) + Ca(OH)₂(s) → 2CaCO₃(s) + 2H₂O(l)
In Words:
Aqueous Calcium Hydrogencarbonate reacts with solid Slaked Lime (Calcium Hydroxide) to form a solid precipitate of Calcium Carbonate and liquid Water.
Question 14.
State the disadvantages of using hard water.
Ans:
One major drawback of hard water is its direct impact on cleaning and household chores. The dissolved calcium and magnesium salts in hard water react with soap to form an insoluble precipitate, often seen as a sticky scum. This reaction prevents soap from lathering properly, meaning you need to use significantly more soap or detergent to get clothes or surfaces clean. This not only leads to wastage of money but also leaves a filmy residue on washed clothes, making them feel rough and look dull. Similarly, this scum can build up on skin and hair, leaving them feeling dry and lackluster.
Another significant disadvantage is the formation of scale, which causes both efficiency and financial problems. When hard water is heated, as in kettles, water heaters, or industrial boilers, the soluble bicarbonates decompose to form insoluble calcium carbonate. This solid deposit, known as scale, coats the inside of pipes and appliances. This scale layer is a poor conductor of heat, forcing boilers to use more energy to heat water, thereby increasing electricity bills. Over time, scale can also clog pipes, reduce water flow, and cause severe damage to appliances, leading to costly repairs or replacements.
Beyond the home, hard water poses a serious problem for industrial operations. Many industries rely on boilers for power generation and manufacturing processes. Scale formation in these large-scale boilers is not just an efficiency issue; it can be a severe safety hazard. The insulating effect of scale can cause the boiler metal to overheat, potentially leading to dangerous explosions. Furthermore, in industries like textiles and dyeing, the minerals in hard water can react with the chemicals used, leading to uneven dyeing and spoilage of fabrics, resulting in inferior product quality and financial losses.
Question 15.
What is soap? For what is it used?
Ans:
What is Soap?
At its core, soap is the original cleaning agent, a product of a fascinating chemical dance between fat and alkali. Imagine combining something oily, like animal tallow or plant-based coconut oil, with a strong, watery solution made from wood ashes or lye (sodium hydroxide). These two opposites don’t just mix; they react in a process called saponification.
This reaction fundamentally transforms the ingredients. The result is a new substance that is neither fat nor alkali. A soap molecule has a unique two-part personality:
- A “head” that is attracted to water (hydrophilic).
- A long “tail” that is repelled by water but attracted to oils and grease (hydrophobic).
This dual nature is the secret to its power.
What is it Used For?
While cleaning is the universal answer, the applications are surprisingly diverse.
1. The Primary Use: Lifting Away Grime
When you wash with soap and water, the hydrophobic tails of the soap molecules burrow into a droplet of grease or a speck of dirt on your skin. The water-loving heads, meanwhile, face outward, surrounding the grime and forming a structure known as a “micelle.” When you rinse, the hydrophilic heads easily follow the water, physically lifting the encapsulated oil and dirt off the surface and washing it away. It doesn’t kill germs outright but evicts them from their hiding places.
2. Beyond Personal Hygiene
Soap’s utility extends far beyond the bathroom:
- Household Cleaning: From scrubbing floors to washing dishes, soap is the foundational ingredient in many cleaning products, effectively removing food residue, soil, and everyday grime.
- Textile Care: Before modern detergents, soap was essential for laundering clothes and fabrics, helping to lift stains and body soils from fibers.
- Lubrication and Release: A simple bar of soap can be rubbed on a sticky drawer slide, a squeaky door hinge, or even along a zipper to help it glide smoothly. It also works as a mild mold release for tasks like concrete work or crafts.
- A Tool for Artisans: Carvers and sculptors sometimes use soap as a soft, workable medium for creating preliminary models or simple artworks.
Question 16.
What is the advantage of a detergent over soap?
Ans:
The primary advantage of detergents over soaps lies in their ability to perform effectively in any type of water, especially hard water. Soap molecules react with the calcium and magnesium salts present in hard water to form an insoluble precipitate commonly known as scum. You’ve likely seen this as a greyish film on clothes after washing or as a ring around the bathtub. This scum is not only difficult to rinse away, leaving fabrics stiff and dull, but it also wastes soap, as a portion of it doesn’t contribute to cleaning at all.
Detergents, on the other hand, are specially engineered synthetic salts of ammonium or sulphonate, which do not form these insoluble compounds with metal ions in hard water. Their cleansing molecules remain active and soluble, allowing them to lather freely and clean efficiently regardless of water hardness. This makes them far more versatile and reliable for household and industrial cleaning.
Furthermore, detergents have a broader chemical range, allowing manufacturers to tailor them for specific purposes. They can be formulated to include additional ingredients like enzymes to break down protein-based stains (like blood or egg), whiteners to make clothes appear brighter, and perfume for a lasting fresh scent. This specialized functionality, combined with their superior performance in all water conditions, is why detergents have largely replaced soaps for most cleaning tasks, particularly laundry.
Question 17.
1. Why does the hardness of water render it unfit for use in a boiler?
2. Why does the hardness of water render it unfit for use in washing purposes.
Ans:
1. Why Hard Water is Unfit for Boiler Use
Using hard water in a boiler is a recipe for inefficiency and damage, primarily due to a problem known as scale formation.
When hard water is heated, the dissolved calcium and magnesium bicarbonate minerals decompose. This process transforms them into insoluble solid compounds, primarily calcium carbonate (lime scale) and magnesium hydroxide. These solids are no longer able to stay dissolved in the water and instead form a hard, cement-like crust on the interior surfaces of the boiler. This crust, or scale, causes a cascade of serious issues:
- Reduced Heat Transfer: Scale is a very poor conductor of heat compared to metal. A layer of scale acts as an insulating blanket, forcing the boiler to consume significantly more fuel to heat the water to the required temperature. This leads to substantial energy waste and higher operational costs.
- Overheating of Boiler Metal: Because the heat from the furnace cannot pass efficiently through the scale and into the water, the boiler metal itself can become dangerously overheated. In steel boilers, this can cause the metal to soften, weaken, and eventually lead to bulging or even catastrophic failure under pressure.
- Blockage of Tubes and Pipes: As scale continues to build up over time, it can severely narrow or completely block water tubes and steam pipes. This restricts water circulation and steam flow, impairing the boiler’s function and creating unsafe pressure conditions.
- Corrosion Promotion: Scale deposits are often uneven and can create localized spots of high stress and concentration cells, which can accelerate the corrosion of the underlying metal, further shortening the boiler’s lifespan.
In essence, the minerals in hard water fundamentally change under heat, creating a destructive insulating layer that wastes fuel, risks dangerous equipment failure, and leads to costly repairs and downtime.
2. Why Hard Water is Unfit for Washing Purposes
Hard water is problematic for washing because it directly impairs the cleaning action of soap and creates undesirable residues.
The core issue lies in a chemical reaction. Soap is typically a sodium salt of fatty acids. When it is added to hard water, the calcium and magnesium ions present do not allow the soap to dissolve and lather properly. Instead, they react with the soap to form an insoluble, sticky precipitate often seen as a grayish-white scum.
This reaction has several negative consequences:
- Waste of Soap: A considerable amount of soap is used up not in cleaning, but in first reacting with the hardness minerals to form this scum. Only after all the calcium and magnesium ions have been precipitated will the soap begin to produce a lather and act as a cleaning agent. This makes washing with hard water remarkably inefficient and expensive.
- Formation of Scum on Fabrics: The insoluble soap curd (the precipitate) is not easily rinsed away. It clings to the fibers of clothing, towels, and fabrics, making them feel rough, stiff, and look dull. This residue also traps dirt particles, causing colors to fade and whites to appear gray or yellow over time.
- Skin and Hair Problems: The same scum that forms on clothes also forms a film on skin and hair. This can clog pores, irritate sensitive skin, and strip away natural oils, leading to dryness and itchiness. For hair, it leaves a coating that makes it look limp, dull, and difficult to manage.
- Bathtub Ring and Stains: The scum is responsible for the familiar “bathtub ring” and the cloudy film left on glassware, shower doors, and sinks after washing, requiring additional scrubbing and harsh cleaners to remove.
Question 18.
1. Explain with equation, what is noticed when permanent hard water is treated with Slaked time
2. Explain with an equation what is noticed when permanent hard water is treated with Washing soda.
Ans:
1. Treatment of Permanent Hard Water with Slaked Lime
Permanent hard water, which contains dissolved chlorides or sulfates of calcium and magnesium, can be treated with slaked lime, which is chemically calcium hydroxide [Ca(OH)₂]. This process is known as the Clark’s process.
When slaked lime is added to the water, it reacts with the soluble calcium and magnesium salts. For example, it reacts with dissolved calcium sulfate. The calcium ions from the calcium sulfate combine with the hydroxide ions from the slaked lime to form an insoluble precipitate of calcium carbonate. Meanwhile, the sulfate ions combine with calcium to form gypsum, which is only slightly soluble. The key point is that the magnesium ions form a precipitate of magnesium hydroxide. These insoluble compounds are what we see as a white, cloudy precipitate.
The reaction can be represented by this equation:
Ca(OH)₂ (aq) + CaSO₄ (aq) → 2CaSO₄ (s) + Mg(OH)₂ (s)
What is noticed is that the initially clear water becomes cloudy or milky as these precipitates form. After allowing the mixture to stand, this white solid settles at the bottom as a sediment, leaving the softened water above, which can then be decanted or filtered.
2. Treatment of Permanent Hard Water with Washing Soda
Permanent hard water can also be effectively treated with washing soda, which is sodium carbonate decahydrate (Na₂CO₃.10H₂O). This method works through a simple double displacement reaction.
Washing soda adds carbonate ions (CO₃²⁻) to the water. These carbonate ions react with the calcium and magnesium ions present in the water. The calcium ions combine with carbonate ions to form insoluble calcium carbonate, while the magnesium ions form insoluble magnesium carbonate. Simultaneously, the sodium ions from the washing soda pair with the chloride or sulfate ions, forming soluble sodium salts that remain dissolved in the water.
The reaction with calcium chloride, for instance, is:
CaCl₂ (aq) + Na₂CO₃ (aq) → CaCO₃ (s) + 2NaCl (aq)
Visually, you would observe the same phenomenon: the formation of a white, cloudy precipitate of calcium carbonate or magnesium carbonate in the water. Once this precipitate settles down, it can be removed, and the remaining water is soft and free from the hardness-causing ions.
Question 19.
Explain the permutit method for softening hard water.
Ans:
The Permutit method is a clever chemical process used to soften hard water, which relies on a man-made substance called Permutit, a type of zeolite. This material is technically known as sodium aluminium orthosilicate. Its special property is that it can easily exchange the sodium ions it contains for other ions present in water. In a practical setup, hard water is passed through a large tank or cylinder that is packed with a bed of these Permutit granules. As the water flows over them, a straightforward ion-exchange reaction occurs.
The heart of the process is a chemical swap. The calcium and magnesium ions, which are the main culprits causing water hardness, are trapped by the Permutit. In their place, the Permutit releases harmless sodium ions into the water. Since sodium salts are highly soluble and do not cause scale formation or interfere with soap, the water that exits the tank is effectively soft. A key advantage of this method is that it doesn’t require any dissolved gases to be removed and it operates smoothly without the need for a pre-boiling step.
However, the Permutit bed doesn’t last forever. After treating a certain volume of hard water, it becomes exhausted. This happens when all the available sodium ions in the Permutit have been swapped out for calcium and magnesium ions. At this point, the material is regenerated by flushing it with a concentrated brine solution, which is rich in sodium ions. This high concentration reverses the reaction, kicking the calcium and magnesium ions off the Permutit and replacing them with sodium ions once again, making the bed ready for another cycle of softening.


