- Defining Air: The chapter probably commences by establishing that air is not a singular chemical substance but rather a heterogeneous mixture composed of several distinct gases. It would underscore the indispensable role of air in sustaining life on Earth.
- Compositional Analysis of Air: This section would detail the principal components of air and their approximate volumetric proportions. The primary focus would likely be on:
- Nitrogen (comprising approximately 78%): emphasizing its prevalence and relative chemical inertness.
- Oxygen (constituting around 21%): highlighting its critical role in the biological process of respiration and the chemical reaction of combustion.
- Carbon Dioxide (present in a small percentage): elucidating its importance in the biological process of photosynthesis and noting its increasing concentration due to anthropogenic activities.
- Trace Gases: mentioning the presence of other gases in minute quantities, such as argon, neon, and helium.
- Variable Components: acknowledging the presence of water vapor and particulate matter (dust) in varying amounts depending on environmental conditions.
- Understanding the Atmosphere: This segment would likely introduce the concept of the Earth’s atmosphere as a protective layer of air enveloping the planet. It might provide a concise overview of the different strata within the atmosphere (such as the troposphere and stratosphere) and their basic characteristics, with a primary emphasis on the troposphere, where terrestrial life and weather phenomena are concentrated.
- Significance of Air and the Atmosphere: This portion would underscore the diverse and crucial functions of air and the atmosphere in supporting life and maintaining the planet’s environmental equilibrium. Key aspects likely covered include:
- Providing oxygen necessary for respiration in most living organisms.
- Supplying carbon dioxide essential for the process of photosynthesis in plants.
- Regulating the Earth’s temperature within a range conducive to life.
- Shielding the Earth’s surface from harmful solar radiation (with a possible brief mention of the ozone layer’s role within the atmosphere).
- Facilitating meteorological phenomena such as wind and precipitation.
- Introduction to Air Pollution: This section would likely introduce the concept of air pollution, defining it as the contamination of the atmosphere by harmful substances. Common sources of air pollutants (such as emissions from vehicles, industrial discharge, and the combustion of fossil fuels) and their fundamental adverse effects on the environment and health might be discussed.
In essence, the chapter aims to establish a foundational comprehension of air as a vital mixture of gases that constitutes the Earth’s atmosphere. It emphasizes the gaseous composition of air, the basic structure of the atmosphere, its critical importance for sustaining life, and an initial understanding of the issue of air pollution.
A MIXTURE OF GASES
EXERCISE — I
Question 1.
Give one use for each of the following inert gases :
(a) argon
(b) helium
(c) neon
(d) radon
(e) krypton
(f) xenon
Ans:
(a) Argon: Employed as a protective atmosphere in welding processes to inhibit unwanted reactions, such as oxidation, between the ambient air and the metals being fused.
(b) Helium: Utilized as a buoyant medium in lighter-than-air craft, including meteorological balloons and airships, owing to its low density and non-combustible nature.
(c) Neon: Applied in the creation of illuminated advertising displays (neon signs), producing a characteristic vibrant reddish-orange glow upon the passage of an electrical discharge.
(d) Radon: In certain medical contexts, utilized in radiotherapy procedures where small, sealed sources containing the gas are introduced into or near cancerous tissues to deliver localized radiation.
(e) Krypton: Incorporated in specific types of high-luminosity lighting systems, such as those used for illuminating airport runways and certain photographic flash units, to enhance light output and efficiency.
(f) Xenon: Employed in the production of high-intensity discharge (HID) automotive headlamps to generate a bright, white light, and also utilized in specialized medical imaging techniques like Xenon-enhanced computed tomography (CT) scans to visualize blood flow and tissue perfusion.
Question 2.
Answer the questions put against each of the following constituents of air :
(a) Nitrogen : Explain its significance for plants and animals.
(b) Oxygen : What is the percentage proportion of oxygen in air ? Why is oxygen called active air.
(c) Carbon dioxide : “Although carbon dioxide plays no role in respiration, all life would come to an end if there is no carbon dioxide in air.” Support this statement with relevant facts.
(d) Water vapours : Explain their role in modifying the earth’s climate.
Ans:
(a) Nitrogen: Plants need it for growth (chlorophyll, proteins, DNA); animals get it from food for proteins and DNA.
(b) Oxygen: About 21% of air. “Active” because it readily reacts in burning and respiration.
(c) Carbon dioxide: Not used in animal respiration, but essential for plant photosynthesis (food and oxygen source for all life) and helps regulate Earth’s temperature.
(d) Water vapours: Act as a greenhouse gas (warming), form clouds (affecting temperature by reflecting sunlight and trapping heat), and cause precipitation (essential for freshwater).
Question 3.
Define the following terms :
(a) pollutants
(b) acid rain
(c) Global warming
(d) smog
Ans:
(a) Pollutants: Harmful substances or energy introduced into the environment.
(b) Acid rain: Unusually acidic precipitation caused by sulfur and nitrogen oxides from burning fossil fuels.
(c) Global warming: Long-term increase in Earth’s average temperature due to increased greenhouse gases from human activities.
(d) Smog: Intense air pollution reducing visibility, a mix of smoke and fog (industrial) or pollutants formed by sunlight reacting with emissions (photochemical).
Question 4.
“Air is a mixture”. Support this statement citing at least three evidences.
Ans:
1. Variable Composition: The proportions of the different gases in air are not constant and can vary depending on location, altitude, humidity, and the presence of pollutants. For instance, the amount of water vapor in the air differs significantly between a desert and a rainforest. Similarly, the concentration of carbon dioxide can be higher in industrial areas or near dense vegetation. If air were a compound, its composition would be fixed and uniform. The fact that we can find varying percentages of its constituents demonstrates it’s a mixture.
2. Individual Properties Retained: The constituent gases of air maintain their individual chemical and physical properties even when mixed. For example:
- Nitrogen remains relatively unreactive.
- Oxygen continues to support combustion and respiration.
- Carbon dioxide can still turn limewater milky.
- Water vapor can condense into liquid water. If air were a compound, the constituent elements would have chemically combined to form a new substance with properties entirely different from the original elements. The fact that the gases in air exhibit their characteristic behaviors indicates they are physically mixed, not chemically bonded.
3. Ease of Separation: The components of air can be separated using physical methods, which is characteristic of mixtures. For example, fractional distillation of liquefied air is used on an industrial scale to separate nitrogen, oxygen, and argon based on their different boiling points. This process involves only physical changes (changes of state) and does not involve breaking chemical bonds. If air were a compound, separating its components would require chemical reactions to break those bonds. The relative ease with which air’s components can be isolated through physical means strongly suggests it is a mixture.
Question 5.
What is air pollution ? What are the harmful effects of sulphur dioxide, nitrogen dioxide and hydrogen sulphide present in the air ?
Ans:
Air pollution: Harmful substances in the air.
Sulfur Dioxide (SO2): Breathing problems, eye irritation, acid rain, forms harmful particles.
Nitrogen Dioxide (NO2): Breathing problems, lung damage, forms smog and ozone, harms environment.
Hydrogen Sulfide (H2S): Rotten egg smell (dangerous at high levels), irritates eyes/nose/throat, nervous system issues, can be deadly.
Question 6.
(a) What are the causes of air pollution ?
(b) Suggest five measures to prevent air pollution.
Answer:
(a) Causes of Air Pollution: Burning fossil fuels, industrial emissions, vehicle exhaust, agriculture, household sources, waste burning, construction, natural events.
(b) Prevention Measures: Use public transport/cycle/walk, switch to clean energy, stricter emission rules, better waste management (reduce/reuse/recycle, no open burning), plant more trees.
Question7.
(a) What is nitrogen-fixation ?
(b) What are the two ways in which nitrogen fixation occurs?
(c) Explain the conversion of nitrogen into nitrates during lightning.
Ans:
(a) Nitrogen-fixation: Converting unusable atmospheric nitrogen (N₂) into usable nitrogen compounds (like ammonia, nitrates) for life.
(b) Two ways: 1. Biological: Microorganisms (bacteria) convert N₂ into ammonia. 2. Physical (Atmospheric): Lightning’s energy converts N₂ and O₂ into nitrogen oxides.
(c) Lightning to Nitrates: Lightning’s energy breaks N₂ and O₂ bonds, forming nitric oxide (NO). NO reacts with more O₂ to form nitrogen dioxide (NO₂). NO₂ dissolves in rainwater to form nitric acid (HNO₃). Nitric acid reacts with soil minerals to form nitrates (NO3−), which plants can absorb.
B. OXYGEN
EXERCISE — II
Question 1.
Name :
(a) The most abundant element in the earth’s crust.
Ans. Oxygen.
(b) A chemical called oxygenated water.
Ans. H2O2 (Hydrogen peroxide)
(c) A metal highly resistant to rusting.
Ans. Tin.
(d) A mixture of oxygen and carbon dioxide used for artificial respiration.
Ans. Carbogen
(e) Two substances from which oxygen can be obtained at a large scale.
Ans. Air, water.
(f) An oxide and a carbonate containing oxygen.
Ans. Mercuric oxide and potassium chlorate.
(g) Two substances which undergo rapid oxidation.
Ans. Sodium, carbon.
Question 2.
(a) Taking hydrogen peroxide, how would you prepare oxygen in the laboratory ?
(b) What is the role of manganese dioxide in the preparation of oxygen ?
(c) Write the balanced chemical equation for the above chemical reaction.
(d) Why is hydrogen peroxide preferred in the preparation of oxygen gas ?
(e) Why is oxygen collected by downward displacement of water ?
(f) What happens when a glowing splinter is introduced in a jar containing oxygen ?
(g) What happens when oxygen gas is passed through alkaline pyrogallol solution ?
Ans:
(a) Oxygen from Hydrogen Peroxide: Add hydrogen peroxide to manganese dioxide in a flask, collect the gas released over water.
(b) Manganese Dioxide’s Role: Catalyst; speeds up the reaction without being used up.
(c) Balanced Equation: 2H2O2(aq)MnO2(s) 2H2O(l)+O2(g)
(d) Why Hydrogen Peroxide Preferred: Safe, easy to control, produces relatively pure oxygen, no harmful byproducts.
(e) Downward Displacement of Water: Oxygen is not very soluble in water, so it can be collected over it by displacing the water downwards.
(f) Glowing Splinter in Oxygen: It reignites and burns brightly.
(g) Oxygen through Alkaline Pyrogallol: Oxygen is absorbed, and the solution turns dark brown or black.
Question 3.
(a) What happens when
- mercuric oxide and
- potassium nitrate are heated ?
(b) Why is potassium chlorate not used for laboratory preparation of oxygen ?
Ans:
(a) Mercuric Oxide & Potassium Nitrate Heated: Both decompose to release oxygen. Potassium nitrate might react with mercury vapor at high temps. Not a preferred method due to mercury’s toxicity and reaction complexity.
(b) Why Not Potassium Chlorate for Oxygen Prep?: Risk of explosion if heated alone or with impurities. Needs high temperature. Can form more unstable potassium perchlorate. Safer alternatives like hydrogen peroxide decomposition exist.
Question 4.
What are oxides ? Give two examples for each of me – tallic and non-metallic oxides.
Ans:
Oxides: Compounds with oxygen and another element (oxygen usually -2 oxidation state).
Metallic Oxides (basic):
- Magnesium Oxide (MgO)
- Calcium Oxide (CaO)
Non-Metallic Oxides (acidic):
- Carbon Dioxide (CO₂)
- Sulfur Dioxide (SO₂)
Question 5.
Name the three types of oxidation processes. In which of these large amount of heat and light energy are produced?
Ans:
- Rapid Oxidation (Combustion): This process entails a swift chemical reaction between a substance and an oxidizing agent, typically oxygen, resulting in the rapid generation of both thermal and luminous energy. This phenomenon is commonly recognized as burning or combustion.
- Slow Oxidation: This refers to a gradual chemical process wherein a substance reacts with oxygen over an extended duration, generally without the conspicuous production of heat or light. A common illustration of this is the formation of rust on iron surfaces.
- Spontaneous Oxidation: This type of oxidation occurs inherently without the need for an external ignition source. Certain materials, under specific environmental conditions, can undergo self-ignition due to a slow oxidation process that gradually generates sufficient thermal energy to reach their auto-ignition temperature.
Question 6.
What do you observe when the following substances are heated and then tested with moist blue and red litmus – paper?
(a) Sulphur
(b) Phosphorus
(b) Calcium
(d) Magnesium
Ans:
(a) Sulphur : Blue litmus turns red.
(b) Phosphorus : Blue litmus turns red.
(c) Calcium : Red litmus turns blue.
(d) Magnesium : Red litmus turns blue.
Question 7.
Complete and balance the following chemical equations.
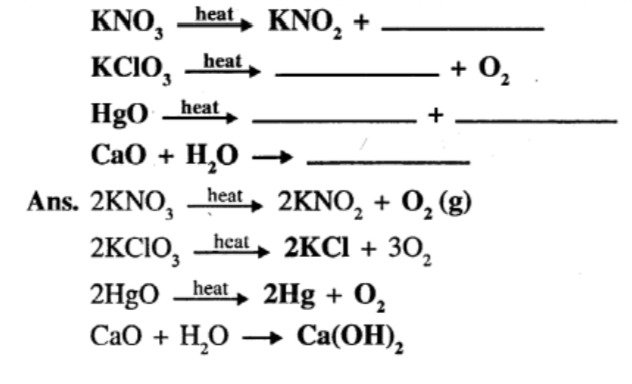
Question 8.
(a) Give four uses of oxygen.
(b) How is oxygen naturally renewed in air ?
Ans:
(a) Four Uses of Oxygen:
1. Breathing (respiration)
2. Burning (combustion)
3. Medical treatment
4. Industrial processes (steel, welding, chemicals)
(b) Natural Renewal of Oxygen: Photosynthesis by plants, algae, and cyanobacteria uses sunlight, water, and carbon dioxide to make food and releases oxygen.
Question 9.
(a) What is rust ?
(b) State at least two ways of prevent rusting.
Ans:
(a) Rust: Reddish-brown hydrated iron(III) oxide (Fe2O3⋅nH2O) formed by iron reacting with oxygen and moisture (electrochemical process).
(b) Rust Prevention (at least two):
1. Protective Layer: Paint, oil, grease, plastic, powder coating to block air and water.
2. Galvanization: Zinc coating corrodes instead of iron (sacrificial protection).
Question 10.
State two differences between : Rusting and burning.
| Feature | Rusting | Burning (Combustion) |
| Speed of Reaction | It is a slow process that can take days, months, or even years to notice. | It is a fast process that occurs almost instantly once the ignition temperature is reached. |
| Energy Release | Energy is released so slowly that no heat or light is observable. | Energy is released rapidly, producing visible heat and light (flames). |
OBJECTIVE TYPE QUESTIONS
1. Fill in the blanks :
(a) ———–is the most abundant inert gas present in air.
Ans : Argon
(b) Oxides of sulphur and nitrogen combine with rain water to form ———–and ———–which cause———-.
Ans : sulphuric acid , nitric acid , acid rain
(c) ———–and CO are the most common air pollutants.
Ans : NO2
(d) ————discovered the oxygen gas.
Ans : Joseph Priestly
(e) Oxygen occupies about ————of air by volume.
Ans : 21%
2. Match the following :
MULTIPLE CHOICE QUESTIONS
1. A fuel when used releases least amount of pollutants in the air.
(a) sulphur dioxide
(b) chlorofluorocarbon
(c) smoke
(d) CNG
2. The natural way of adding oxygen to air which involves green plants is called
(a) photosynthesis
(b) respiration
(c) burning
(d) dissolution
3. Which one of the following is most likely to be corroded?
(a) a stainless steel cup-board
(b) a galvanised iron bucket
(c) an iron hammer
(d) a tin plated iron box
4. The process by which oxidation of food in our body takes place is
(a) photosynthesis
(b) respiration
(c) decomposition
(d) combustion


