1. Introduction & Preparation
Ammonia is a colorless, pungent-smelling gas, lighter than air and highly soluble in water. In the laboratory, it is prepared by heating a mixture of an ammonium salt (like Ammonium Chloride) with a strong base (like Calcium Hydroxide).
Equation: 2NH₄Cl + Ca(OH)₂ → CaCl₂ + 2H₂O + 2NH₃
It is collected by downward displacement of air because it is lighter than air.
2. Manufacture: Haber’s Process
Ammonia is manufactured industrially by the Haber’s Process. Nitrogen from the air and hydrogen from natural gas are combined under specific conditions:
Temperature: ~450°C
Pressure: ~200 atm
Catalyst: Finely divided Iron
The reaction is reversible and exothermic: N₂(g) + 3H₂(g) ⇌ 2NH₃(g) + Heat
3. Properties
Physical: It is highly soluble in water, forming ammonium hydroxide (NH₄OH), which is alkaline and turns red litmus blue.
Chemical:
Basic Nature: It is a strong base and reacts with acids to form ammonium salts. (e.g., NH₃ + HCl → NH₄Cl)
Combustibility: It burns in oxygen with a greenish-yellow flame to form nitrogen and water.
As a Reducing Agent: It reduces hot metal oxides like copper(II) oxide to the metal.
4. Tests for Ammonia Gas
Its pungent smell is a key indicator.
It turns moist red litmus paper blue.
It forms dense white fumes when a glass rod dipped in concentrated HCl is brought near it.
5. Uses
Mainly used in the production of fertilizers (like Urea, Ammonium nitrate).
To manufacture nitric acid (Ostwald process).
As a refrigerant.
In the production of cleaning agents and dyes.
EXERCISE
1) Draw a labelled diagram and give a balanced equation for the lab. Preparation of ammonia. Also state physical properties of ammonia.
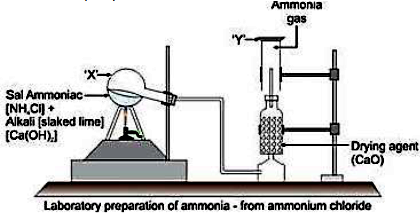
Ans: Balanced Chemical Equation:
Reaction : 2NH4Cl + Ca(OH)2 —>CaCl2 + 2H2 + 2NH3
Physical Properties of Ammonia:
- State: Colourless gas.
- Odour: Pungent, characteristic choking smell.
- Taste: Sharp taste.
- Density: Lighter than air.
- Solubility: Highly soluble in water, forming aqueous ammonia (NH₄OH).
- Liquefaction: Easily liquefied under pressure to a colourless liquid.
- Refrigerant: Liquid ammonia has a high latent heat of vaporization, making it a good refrigerant.
2) (a) Is ammonia more dense or less dense than air?
(b) What property of ammonia is demonstrated by the fountain experiment?
(c) Write the balanced equation for the reaction between ammonia and sulphuric acid
Ans: (a) Ammonia is less dense than air.
(b) The fountain experiment demonstrates the high solubility of ammonia in water.
(c)Balanced equation for the reaction between ammonia and sulphuric acid is: 2NH3 + H2SO4 (NH4)2SO4
3)Pick the odd member from the list giving reasons:
(a) Ammonia, sulphur dioxide, hydrogen chloride, carbon dioxide.
(b) Copper oxide, aluminium oxide, sodium oxide, magnesium oxide.
Ans: (a) Ammonia is basic in nature. (b) Copper oxide because CuO is less reactive can be reduced by C, CO or by hydrogen whereas Al2O3, Na2O, MgO are reduced by electrolysis.
4) The following reactions are carried out:
A: Nitrogen + metal compound X.
B: X + water ammonia + another compound
C: Ammonia + metal oxide metal + water + N2 One metal that can be used for reaction A is magnesium.
(a) write the formula of the compound X formed
(b) write the correctly balanced equation for reaction B where X is the compound formed.
(c) what property of ammonia is demonstrated by reaction C?
Ans: (a) Formula of compound X:
Mg₃N₂ (Magnesium nitride)
(b) Balanced equation for reaction B:
Mg₃N₂ + 6H₂O → 3Mg(OH)₂ + 2NH₃
(c) Property of ammonia demonstrated in reaction C:
Ammonia acts as a reducing agent (it reduces the metal oxide to metal).
5) Ammonium salts decompose on heating. What other property do ammonium salts have in common?
Ans: Reducing property.
6) State what you observe when a piece of moist red litmus paper is placed in a gas jar of ammonia.
Ans: Observation:
When a piece of moist red litmus paper is placed in a gas jar of ammonia, the paper will slowly turn from red to blue.
Reasoning:
This change occurs because ammonia gas is highly soluble in water. The moisture on the litmus paper dissolves the ammonia, forming an alkaline solution called ammonium hydroxide.
Ammonia (NH₃) + Water (H₂O) → Ammonium Hydroxide (NH₄OH)
This ammonium hydroxide solution is basic in nature. Since red litmus paper is used to test for alkalinity, it undergoes a characteristic colour change, turning blue in the presence of this base.
It’s important to note that the litmus paper must be moist for this reaction to work effectively, as dry ammonia gas itself does not cause the colour change on dry litmus paper.
7) A gas ‘P’ gives dense white fumes with chlorine. Its aqueous solution gives a blue colour with copper (II) hydroxide (a) Name the gas P. (b) Give its formula (c) Give three uses of P.
Ans: (a) Gas P is Ammonia.
(b) Its chemical formula is NH₃.
(c) Three uses of ammonia:
Making fertilizers such as urea.
Acting as a refrigerant in industrial cooling systems.
Manufacturing cleaning products for household use.
8) Ammonia solution in water gives a blue precipitate when it combines with a solution of copper salt. The blue precipitate further dissolves in excess of ammonia solution to give azure blue solution. Explain with equation.
Ans: When ammonia solution is added to a solution containing copper ions (like Cu²⁺ from CuSO₄), a pale blue precipitate of copper(II) hydroxide is formed.
Reaction 1: Formation of Blue Precipitate
CuSO4(aq)+2NH4OH(aq)→Cu(OH)2(s)+(NH4)2SO4(aq)CuSO 4 (aq)+2NH 4 OH(aq)→Cu(OH) 2(s)+(NH 4 ) 2SO 4 (aq)
(The pale blue precipitate is Cu(OH)₂.)
When excess ammonia is added, the precipitate dissolves because ammonia molecules act as ligands and form a soluble complex ion with copper.
Reaction 2: Dissolution in Excess Ammonia
Cu(OH)2(s)+4NH3(aq)→[Cu(NH3)4]2+(aq)+2OH−(aq)Cu(OH) 2 (s)+4NH 3(aq)→[Cu(NH 3 ) 4 ] 2+ (aq)+2OH −(aq)
This forms the deep azure blue complex tetraamminecopper(II) ion.
9) How do you prove that NH3 contains nitrogen and hydrogen?
Ans: To prove that ammonia (NH₃) contains nitrogen and hydrogen, you can perform the following simple tests:
1. Test for Hydrogen:
Hold a dry, inverted glass jar over ammonia gas.
Insert a burning splint into the jar.
The hydrogen in ammonia will burn, often with a pale yellow flame, and you will hear a “pop” sound.
2. Test for Nitrogen:
Gently heat ammonia gas with solid copper(II) oxide in a hard glass test tube.
The ammonia reduces the copper(II) oxide, and the nitrogen present is released as nitrogen gas.
The nitrogen gas can be identified as it extinguishes a burning splint.
10) Give reasons for the following:
(a) Liquid ammonia is used as a refrigerant in ice plants.
(b) Aqueous solution of ammonia is used for removing grease stains from woolen clothes
(c) Aqueous solution of ammonia gives a pungent smell
(d) Aqueous solution of ammonia conducts electricity
Ans: (a) Liquid ammonia as a refrigerant
It has a very high latent heat of vaporization, absorbing large amounts of heat while evaporating, causing effective cooling. It is also cheap and residue-free.
(b) Ammonia solution for removing grease stains
Ammonia solution is alkaline and reacts with grease, converting it into a soluble soap-like substance that can be easily washed off without harming woolen fibers.
(c) Pungent smell of ammonia solution
The smell arises because ammonia gas (NH₃) escapes from the solution into the air, which our nose detects as pungent.
(d) Electrical conduction by ammonia solution
Ammonia in water forms ammonium ions (NH₄⁺) and hydroxide ions (OH⁻), and the movement of these ions allows the solution to conduct electricity.
11) Copy and complete the following equations.
(a) AIN + H2O
(b) 2NH3 + 3PbO
(c) NH3 + 3CI2
(d) NH3 + CO2
(i) which property of ammonia is illustrated by equation (c) ?
(ii) what important fertilizer is prepared from equation (d) ?
Ans: (a) AlN + 3H2O—> Al(OH)3 + NH3
(b) 2NH3 + 3PbO —>3Pb + 3H2O + N2
(c) 8NH3 + 3Cl2—> N2 + 6NH4Cl
(d) 2NH3 + CO2 —> NH2CONH2 + H2O
(i) The property illustrated by equation (c) is the reducing property of ammonia.
(ii) The important fertilizer prepared from the reaction in equation (d) is Urea.
12) Correct the following:
(a) A reddish brown precipitate is obtained when ammonium hydroxide is added to ferrous sulphate.
(b) Liquid ammonia is a solution of NH3
(c) Finely divided platinum is used in Haber process
(d) Conc. H2SO4 is a drying agent for NH3
(e) Ammonium salts, on heating, decompose to give ammonia.
Ans: (a) True – Ammonium hydroxide with ferric sulphate gives a reddish-brown precipitate of ferric hydroxide.
(b) True – Liquor ammonia is an aqueous solution of ammonia (NH₃) in water.
(c) False – Finely divided iron is used as a catalyst in the Haber process, not iron as a reactant in bulk.
(d) True – Concentrated H₂SO₄ can dry SO₂, but it cannot dry NH₃ because ammonia reacts with sulfuric acid.
(e) True – Ammonium salts decompose on heating to give ammonia and the corresponding acid.
13) What do you observe when ammonium hydroxide is added to the aqueous solution of: (a) FeSO4 (b) Iron (III) chloride, (c) Lead nitrate (d) Zinc nitrate?
Ans: (a) With FeSO₄ (Ferrous Sulphate)
A dirty green precipitate (gelatinous) of ferrous hydroxide is formed.
Chemical Reason: FeSO₄ reacts with NH₄OH to form insoluble Fe(OH)₂.
(b) With Iron (III) chloride
A reddish-brown precipitate of ferric hydroxide is formed.
Chemical Reason: FeCl₃ reacts with NH₄OH to form insoluble Fe(OH)₃.
(c) With Lead nitrate
A chalky white precipitate of lead hydroxide is formed.
Chemical Reason: Pb(NO₃)₂ reacts with NH₄OH to form insoluble Pb(OH)₂.
(d) With Zinc nitrate
A white precipitate of zinc hydroxide is formed, which dissolves in excess ammonium hydroxide to form a clear, colorless solution.
Chemical Reason: The initial Zn(OH)₂ precipitate reacts with excess NH₃ from NH₄OH to form a soluble complex ion, [Zn(NH₃)₄]²⁺.
14) Why is ammonium hydroxide used in qualitative analysis? Give two equations to justify your answer
Ans: Ammonium hydroxide (NH4OH) is used in qualitative analysis primarily because it provides OH−− ions and NH3 molecules in solution, allowing it to:
- Precipitate hydroxides of certain metal ions.
- Form complex ions with other metal ions, dissolving their hydroxides.
This selective precipitation and dissolution help in the identification and separation of cations in a mixture.
Two Justifying Equations:
1. Precipitation of Aluminium Hydroxide (Group III Analysis):
FeSO4 + 2NH4OH (NH4).2SO4 + Fe (OH)2
This insoluble hydroxide is used to identify the presence of Al3+3+ ions.
2. Dissolution of Copper Hydroxide by Complex Formation (Group IV Analysis):
FeCl3 + 3NH4OH 3NH4Cl + Fe (OH)3
The formation of a deep blue complex ion confirms the presence of Cu2+2+ ions.
15) Give a chemical test to distinguish between the following:
(a) Ammonium chloride and sodium chloride
(b) Ferric salt and ferrous salt
(c) Sodium sulphate and ammonium sulphate
Ans: (a) Heating NH₄Cl vs NaCl
NH₄Cl sublimes on strong heating, producing dense white fumes of NH₃ and HCl that recombine into a white powdery solid on cooler parts.NaCl does not sublime or produce white fumes on heating.
(b) Reaction with NH₄OH
Ferrous salt (Fe²⁺) gives a dirty green precipitate of Fe(OH)₂.
Ferric salt (Fe³⁺) gives a reddish-brown precipitate of Fe(OH)₃.
(c) Warming with NaOH
(NH₄)₂SO₄ liberates NH₃ gas when warmed with NaOH.
Na₂SO₄ does not release NH₃ gas.
16) Give balanced equations for the following conversation:
(a) Ammonia to nitrogen using an acidic gas,
(b) Ammonia to brown gas,
(c) Ammonia to nitrogen trichloride
(d) Ammonia solution to an amphoteric hydroxide
(e) A nitride of a trivalent metal to ammonia
(f) Lead oxide to lead.
Ans: Balanced equations : (a) 8NH3 + 3Cl2—> N2 + 6NH4Cl
(b) 4NH3 + 5O2 0 Pt 800C —> 4NO + 6H2O +Heat
2NO+O2 —> 2NO2 Brown gas
(c) NH3 +3Cl2 —> 3HCl +NCl3
(d) AlCl3 +3NH4OH —> 3NH4Cl + Al(OH)3
(e) AlN + 3H2O —> Al(OH)3 + NH3
(f) 3PbO +2NH3 —> 3Pb + 3H2O +N2
17) (a) explain catalytic oxidation of ammonia
(b) Give two reactions to show reducing properties of ammonia.
Ans: (a) Catalytic Oxidation of Ammonia
In this process, a mixture of ammonia and oxygen is passed over a heated catalyst (usually platinum or rhodium at around 800-900°C). Ammonia is oxidized to nitric oxide (NO) and water. This is the primary step in the industrial manufacture of nitric acid (Ostwald process).
The balanced chemical equation is:
4NH₃ + 5O₂ → 4NO + 6H₂O
(b) Reducing Property of Ammonia
Ammonia acts as a reducing agent because it can be oxidized, often by strong oxidizing agents. Two reactions showing this are:
With Copper(II) Oxide:
When ammonia gas is passed over heated black copper(II) oxide, it reduces it to reddish-brown copper, and itself is oxidized to nitrogen gas.
3CuO + 2NH₃ → 3Cu + N₂ + 3H₂O
With Chlorine Gas:
Ammonia reacts violently with chlorine, reducing it to hydrogen chloride (forming white fumes of ammonium chloride) and itself being oxidized to nitrogen gas.
3Cl₂ + 2NH₃ → N₂ + 6HCl (The HCl then reacts with excess NH₃ to form NH₄Cl fumes).
18) Choose the correct word or phrase from the brackets to complete the following sentences and write balanced equations for the same.
(i) ammonium chloride is a soluble salt prepared by ………………. [precipitation, neutralization]
(ii) when ammonium chloride is heated, it undergoes …………….. [thermal decomposition/ dissociation].
(iii) Heating ammonium chloride with sodium hydroxide produces …………. [ammonia, nitrogen]
Ans: (i) Neutralization NH3 + HCl —> NH4Cl
(ii) Thermal dissociation NH4Cl —> NH3 + HCl
(iii) Ammonia NH4Cl + NaOH—> NH3 + NaCl + H2O
19) Name:
(a) the gas which is prepared by Haber process,
(b) two gases which give dense white fumes with ammonia,
(c) one salt of ammonia in each case which is used in: (i) dry cell (ii) explosive (iii) medicine.
(d) an acidic gas which reacts with a basic gas liberating a neutral gas,
(e) a metallic chloride soluble in ammonium hydroxide,
(f) the gas obtained when ammonia burns in an atmosphere of oxygen without any catalyst
(g) a nitride of a divalent metal which reacts with warm water liberating ammonia (h) an amphoteric oxide reduced by the basic gas.
(i) a white salt produced by an acid gas and a basic gas.
Ans: (a) Ammonia
(b) Hydrogen chloride and chlorine gas.
(c) (i) Ammonium chloride (ii) Ammonium nitrate (iii) Ammonium carbonate (d) Acidic gas: HCl Basic gas: Ammonia Neutral gas: NH4Cl
(e) Silver chloride
(f) Nitrogen
(g) Magnesium nitride
(h) Lead oxide
(i) Ammonium chloride
20) When ammonium hydroxide is added to solution B, a pale blue precipitate is formed. This pale blue precipitate dissolves in excess ammonium hydroxide giving an inky blue solution. What is the cation [positive ion] present in solution B? what is the probable colour of solution B.
Ans: The cation present in solution B is Copper (Cu²⁺).The pale blue precipitate is copper(II) hydroxide, which dissolves in excess NH₄OH to form the deep blue complex ion [Cu(NH₃)₄]²⁺.The probable colour of solution B is pale blue, due to the presence of hydrated copper(II) ions.
21) When an ammonium salt is warmed with a sodium hydroxide solution, ammonia gas is evolved. State three ways in which you could identify this gas.
Ans:
- Litmus test – Ammonia turns damp red litmus paper blue.
- Characteristic smell – It has a distinctive pungent or choking smell.
- Hydrogen chloride test – It forms white fumes of ammonium chloride with concentrated hydrochloric acid.
22) Complete the following equations. What property of ammonia is illustrated by the reaction in (b)?
(a) Mg3N2 + 6H2O —>
(b) 2NH3 + 3CuO —>
(c) 8NH3 +3Cl2 —>
(d) 4 NH3 +5O2 —>
What important process starts with the reaction in (d) above? Name the catalyst used.
Ans: (a) Mg3N2 + 6H2O 3Mg(OH)2 + 2NH3
(b) 2NH3 + 3CuO 3Cu + 3H2O + N2 Ammonia acts as a reducing agent. It reduces metallic oxide to give metals, water vapour and nitrogen
(c) 8NH3 +3Cl2 N2 + 6NH4Cl
(d) 4 NH3 +5O2 6H2O + 4NO +Heat
23) A gas ‘A’ reacts with another gas ‘B’ in the presence of a catalyst to give a colourless gas ‘C’. the gas ‘C’ when comes in contact with air produces a brown gas ‘D’. The solution of ‘A’ in water turns red litmus blue. Explain the observations.
Ans: Step 1: Identify gas A
A’s aqueous solution turns red litmus blue → A is basic in nature → likely Ammonia (NH₃).
Step 2: Reaction of A (NH₃) with B in presence of a catalyst
NH₃ reacts with O₂ in presence of platinum catalyst:
4NH3+5O2→Pt4NO+6H2O4NH 3 +5O 2Pt4NO+6H 2 O
So, B = O₂, C = NO (colourless gas).
Step 3: Gas C (NO) with air gives brown gas D
NO reacts with O₂ in air:
2NO+O2→2NO2
NO₂ is brown → D = NO₂.
Final answer:
A = NH₃, B = O₂, C = NO, D = NO₂.
NH₃ dissolves in water to form NH₄OH (basic, turns red litmus blue).
24) Name the common refrigerant. How does it deplete the ozone layer?
Ans:
- A common ozone-depleting refrigerant is CFC-12, often called Freon.
- It harms the ozone layer through a series of events:
- CFCs are stable and rise intact into the upper atmosphere (the stratosphere).
- There, strong UV radiation from the sun breaks them apart, releasing chlorine atoms.
- These chlorine atoms then break apart ozone (O₃) molecules.
- A single chlorine atom can destroy thousands of ozone molecules, creating a thin spot, or “hole,” in the ozone layer.
- This allows more harmful UV radiation to reach the Earth.
25) (a) What is the alternative of chlorofluoro carbon? (b) State the advantages of using ammonia as refrigerant?
Ans: (a) Alternative to Chlorofluorocarbon (CFC):
Hydrofluorocarbons (HFCs) and natural refrigerants like hydrocarbons (e.g., R-600a or isobutane) are common alternatives.
(b) Advantages of Using Ammonia as a Refrigerant:
High Efficiency: It has excellent heat transfer properties, leading to lower energy consumption.
Low Cost: It is significantly cheaper than most synthetic refrigerants.
Environmentally Friendly: It has zero Ozone Depletion Potential (ODP) and zero Global Warming Potential (GWP).
Easy Leak Detection: Its strong, pungent odor makes even small leaks easy to detect.
26) Ammonia is a good refrigerant but it shows some disadvantages when used as a refrigerant. State the disadvantages.
Ans:
- Toxic: Ammonia is poisonous and can be hazardous if a leak occurs in an enclosed space.
- Flammable: It is mildly flammable at certain concentrations in air, posing a fire risk.
- Incompatible with Copper: It reacts with copper and its alloys, so the entire system must be made from steel or other compatible metals, increasing cost.
- Irritating Odor: While the strong smell helps in leak detection, it is very pungent and irritating to the eyes and respiratory system.
27) Name a compound prepared by ammonia and is used as: (a) Explosive, (b) fertilizers (c) Medicine (d) laboratory reagent
Ans: (a) Explosive: Ammonium Nitrate
Ammonium nitrate’s dual use comes down to the speed of its reaction. In explosives, a detonator triggers a near-instantaneous decomposition, releasing a massive volume of gas that creates a destructive shockwave. This contrasts with its fertilizer use, where it slowly releases nitrogen to nourish plants.
(b) Medicine: Ammonium Carbonate
Historically, ammonium carbonate was the key component in smelling salts. It releases ammonia gas, whose sharp, irritating smell triggers an involuntary reflex. This causes a person who has fainted to take a sudden, deep breath, often jolting them back to consciousness.
(c) Fertilizers: Ammonium Sulphate
Ammonium sulphate is a vital fertilizer because it efficiently delivers two essential nutrients to plants. It provides nitrogen, which is fundamental for building proteins and chlorophyll, and sulphur, which is crucial for forming enzymes and vitamins.
(d) Laboratory Reagent: Ammonia Solution
In the lab, ammonia solution is valued as a versatile weak base. It is used to identify metal ions by forming characteristic precipitates, to clean glassware by dissolving grease, and as a reagent in various chemical synthesis reactions.
28) Ammonia is used in the Ostwald process, (a) Give the sources of reactants used in this process. (b) Name the catalyst used in the process (c) Name the oxidizing agent used in this process (d) What is the ratio of ammonia and air taken in this process? (e) Why is quartz used in this process?
Ans: (a) Sources of reactants:
Ammonia (NH₃): Produced industrially via the Haber process from nitrogen (from air) and hydrogen (from natural gas).
Oxygen (O₂): Sourced directly from air.
(b) Catalyst:
Platinum-Rhodium gauze.
(c) Oxidizing agent:
Oxygen (from air).
(d) Ratio of ammonia and air:
Approximately 1 volume of ammonia to 10 volumes of air.
(e) Why quartz is used:
Quartz (silica) is used for the construction of the oxidation chamber because it can withstand the very high reaction temperature (around 800-900°C) without softening or reacting with the gases.
29) Write the equation for the action of heat on: (a) Ammonium chloride (b) Ammonium nitrate State whether each reaction is an example of thermal decomposition or thermal dissociation.
Ans: (a) Ammonium chloride NH4Cl —> NH3 + HCl
(b) Ammonium nitrate NH4NO3 —> N2O + 2H2O
Question 1(2003): 1. (a) Write the equation for the formation of ammonia by the action of water on magnesium nitride (b) How is ammonia collected? (c) why is ammonia not collected over water? (d) Which compound is normally used as a drying agent for ammonia?
Ans: (a) Formation of ammonia from magnesium nitride and water:
Mg₃N₂ + 6H₂O → 3Mg(OH)₂ + 2NH₃
(b) Collection of ammonia:
In essence, this technique doesn’t fight ammonia’s nature but works with it. By understanding its inherent lightness, we can guide the gas exactly where we want it, making downward displacement a beautifully simple and effective way to collect ammonia in a laboratory setting.
(c) Reason for not collecting over water:
Ammonia is highly soluble in water. If collected over water, it would dissolve and no gas would be collected.
(d) Drying agent for ammonia:
Quicklime (Calcium oxide, CaO) is normally used. Concentrated sulphuric acid or anhydrous calcium chloride cannot be used as they react with ammonia.
Question 1(2004): (a) Write the equation for the reaction in Haber process that forms ammonia. (b) state the purpose of liquefying the ammonia produced in the process.
Ans: (a) Equation for the reaction in the Haber process
The Haber process involves the direct combination of nitrogen gas and hydrogen gas under specific conditions to form ammonia. The balanced chemical equation for this reversible reaction is:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g)
(b) Purpose of liquefying the ammonia produced
The primary purpose of liquefying the ammonia gas is to separate it easily from the unreacted nitrogen and hydrogen gases.
In the reaction vessel, only a fraction of the nitrogen and hydrogen gases convert into ammonia. By cooling the gaseous mixture and applying pressure, the ammonia gas condenses into a liquid, while the unreacted nitrogen and hydrogen remain as gases. This liquid ammonia is then simply drained off. The unreacted gases are recycled back into the reaction chamber to be used again, which improves the overall efficiency and yield of the process.
Question 1(2005): (a) which feature of ammonia molecule leads to the formation of the ammonium ion when ammonia dissolves in water? (b) Name the other ion formed when ammonia dissolves in water. (c) Give one test that can be used to detect the presence of the ion produced in (b)
Ans: (a) The lone pair of electrons on the nitrogen atom.
The ammonia molecule (NH₃) has a nitrogen atom at its center. Nitrogen has five valence electrons, three of which are bonded to hydrogen atoms. This leaves a pair of electrons that is not shared, known as a lone pair. This lone pair is highly attractive to a hydrogen ion (H⁺). When ammonia dissolves in water, a water molecule (H₂O) can donate a H⁺ ion. The nitrogen atom uses its lone pair to form a new covalent bond with this H⁺, resulting in the formation of the ammonium ion (NH₄⁺).
(b) Hydroxide ion (OH⁻).
When ammonia accepts a proton (H⁺) from a water molecule, the remaining part of that water molecule becomes a hydroxide ion (OH⁻). This is why ammonia solutions in water are also called ammonium hydroxide and are basic in nature.
(c) The turning of red litmus paper to blue.
The presence of the hydroxide ion (OH⁻) makes the solution basic or alkaline. A simple and common test for this is to use litmus paper. When a strip of red litmus paper is dipped into the ammonia solution, the hydroxide ions cause the paper to turn from red to blue, confirming the basic nature of the solution and thus the presence of OH⁻ ions.
Question 2(2005): Write the equations for the following reactions which result in the formation of ammonia. (a) A mixture of ammonium chloride and slaked lime is heated. (b) Aluminium nitride and water.
Ans: (a) 2NH4Cl + Ca(OH)2 → CaCl2 + 2H2O + 2NH3
(b) AlN + 3H2O → Al(OH)3 + NH
Question 1(2006) State what is observed when: Excess of ammonia is passed through an aqueous solution of lead nitrate.
Ans: Initially, a white precipitate of lead hydroxide is formed. However, as an excess of ammonia is passed through the solution, this white precipitate does not dissolve.
This is in contrast to many other metal ions (like copper) which form complexes soluble in excess ammonia. The lead hydroxide precipitate remains insoluble because lead does not form a stable ammine complex under these aqueous conditions.
In summary:
You would observe the formation of a white precipitate which persists even when excess ammonia is added.
Question 1(2007): (a) Of the two gases, ammonia and hydrogen chloride which is more dense? Name the method of collection of this gas. (b) Give one example of a reaction between the above two gases which produces a solid compound.
Ans: (a) Between ammonia and hydrogen chloride, hydrogen chloride gas is more dense.
Explanation:
The density of a gas is directly proportional to its molecular mass. Ammonia has a molecular mass of 17 u (N=14, H=1×3), while hydrogen chloride has a molecular mass of 36.5 u (H=1, Cl=35.5). Since hydrogen chloride has a higher molecular mass, it is denser than ammonia.
Method of Collection:Hydrogen chloride gas, being denser than air and highly soluble in water, is collected by the method of Upward Displacement of Air.
(b) When ammonia gas and hydrogen chloride gas come into contact with each other, they react to form a white solid compound known as Ammonium Chloride.
The chemical reaction is:
NH₃ (g) + HCl (g) → NH₄Cl (s)
This is famously demonstrated in the laboratory as the “ammonia fountain” or “white smoke” experiment, where dense white fumes of ammonium chloride are seen when the two gases meet.
Question 2(2007): Write a balanced equation for a reaction in which ammonia is oxidized by: (a) a metal oxide (b) a gas which is not oxygen
Ans: Balanced equation:
(a) 2NH3 + 3CuO → 3Cu + 3H2O + N2
(b) 2NH3 + 3Cl2 → N2 + 6HCl
Question 1(2008): Write equation for the following: Aluminum nitride and water.
Ans: Equation: AlN + 3H2O → Al(OH)3 + NH
Question 2(2008): Choose the correct from the following: Ammonia can be obtained by adding water to A : Ammonium chloride B : Ammonium nitrite, C : Magnesium nitride D : Magnesium nitrate
Ans: Magnesium Nitride
INTEXT 1
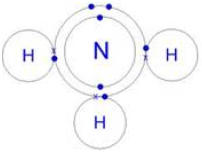
1) State the type of bonding present in ammonia, show by a diagram.
Ans: 1. Covalent Bonding (Primary)
Within a single ammonia molecule, the nitrogen atom shares three of its electrons with three hydrogen atoms. This sharing of electron pairs forms strong covalent bonds, holding the atoms together.
(The three lines between N and each H represent shared electron pairs, i.e., covalent bonds.)
2. Hydrogen Bonding (Intermolecular)
Between different ammonia molecules, a weak attractive force called hydrogen bonding occurs. The highly electronegative nitrogen atom attracts the bonding electrons, making the molecule polar. The hydrogen atoms carry a partial positive charge (δ+) and the nitrogen a partial negative charge (δ-). The attraction between δ+ (H) of one molecule and δ- (N) of another is a hydrogen bond.
2) Name the different forms of ammonia.
Ans: The main forms of ammonia are:
- Gaseous ammonia (NH₃)
- Liquid ammonia (NH₃)
- Ammonium ion (NH₄⁺), found in salts like ammonium chloride.
3) What is the formula of liquid ammonia? Account for the basic nature of this compound.
Ans: The formula for liquid ammonia is NH₃.
It is basic because the nitrogen atom in ammonia has a lone pair of electrons. This lone pair can accept a proton (H⁺) from water. This reaction produces hydroxide ions (OH⁻), which make the solution basic.
NH₃ + H₂O ⇌ NH₄⁺ + OH⁻
4 )Ammonia gas can be prepared by warming an ammonium salt with caustic alkali. Give two equations.
Ans:
- (NH4)2SO4 + 2NaOH —> Na2SO4 + 2H2O +2NH3
- (NH4)2SO4 + 2NaOH —> K2SO4 + 2H2O +2NH
5)(a) Write a balanced chemical equation for the lab preparation of ammonia. (b) How is ammonia dried and collected in the laboratory? (c) Ammonia cannot be collected over water. Give reason.
Ans: (a) Balanced chemical equation for the lab preparation of ammonia:
A common laboratory preparation uses ammonium chloride and calcium hydroxide:
2NH4Cl + Ca(OH)2 CaCl2 + 2H2O + 2NH3
(b) How is ammonia dried and collected?
Drying: Ammonia gas is passed through a drying tower containing Quicklime (CaO). Concentrated sulphuric acid or anhydrous calcium chloride cannot be used because they react with ammonia.
Collection: It is collected by downward displacement of air. This method is used because ammonia is lighter than air.
(c) Reason why ammonia cannot be collected over water:
Ammonia is highly soluble in water. If collected over water, it would dissolve rapidly in the water instead of collecting as a gas.
6) Name a drying agent for ammonia. Why are other drying agents such as P2O5 and CaCl2 not used?
Ans: A suitable drying agent for ammonia is Quicklime (CaO).
Other drying agents like P₂O₅ and CaCl₂ are not used because:
- P₂O₅ is acidic and reacts chemically with basic ammonia.
- CaCl₂ forms an addition compound with ammonia (CaCl₂·8NH₃), so it cannot dry the gas effectively.
7) A substance ‘A’ was heated with slaked lime and a gas ‘B’ with a pungent smell was obtained. Name the substances A and B and give a balanced equation. Name the substances A and B and give a balanced equation.
Ans: The substance A is Ammonium chloride and ‘B’ is Ammonia.
Reaction: 2NH4Cl + Ca(OH)2 → CaCl2 + 2H2O + 2NH3
8) Ammonia is manufactured by Haber process.
(a) Under what condition do the reactants combine to form ammonia? Give a balanced equation for the reaction.
(b) In what ration by volume, are the above gases used?
(c) state one possible source of each reactant used in the Haber process.
(d) state whether the formation of ammonia is promoted by the use of high pressure or low pressure?
(e) Mention two possible ways by which ammonia produced is removed from unchanged gases
(f) What is the function of:
(i) finely divided iron (ii) molybdenum in the above process?
Ans: (a) The reactants, nitrogen and hydrogen, combine under conditions of high pressure (around 200 atm), moderate temperature (around 450°C), and in the presence of an iron catalyst.
Balanced Equation:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g)
(b) The gases are used in the volume ratio of Nitrogen : Hydrogen = 1 : 3.
(c)Nitrogen: From the air (by fractional distillation of liquid air).
Hydrogen: From natural gas (methane) by steam reforming (CH₄ + H₂O → CO + 3H₂).
(d) The formation of ammonia is promoted by the use of high pressure because the reaction involves a decrease in the number of gas molecules.
(e) Two ways to remove ammonia are:
Liquefaction: The gas mixture is cooled, causing the ammonia to condense into a liquid while the unreacted nitrogen and hydrogen remain gaseous.
Dissolution: The gas mixture is passed through water, where ammonia dissolves to form ammonium hydroxide, leaving the other gases behind.
(f)(i) Finely divided iron acts as a catalyst to speed up the rate at which the reaction reaches equilibrium.
(ii) Molybdenum acts as a promoter to increase the efficiency of the iron catalyst.
9) Given reasons: (a) Ammonium compounds do not occur as minerals
(b) Ammonium nitrate is not used in the preparation of ammonia
(c) Conc H2SO4 is a good drying agent, yet it is not used to dry NH3.
Ans: (a) Ammonium compounds are highly soluble in water. Therefore, they are easily washed away from rocks and soil by rain and do not persist as minerals.
(b) Ammonium nitrate (NH₄NO₃) is not used because it is explosive in nature. When heated, it can decompose violently, making the process dangerous and unsuitable for the safe preparation of ammonia gas.
(c) Concentrated H₂SO₄ is a strong acid, while ammonia (NH₃) is a base. They react chemically to form ammonium sulfate, [(NH₄)₂SO₄]. Therefore, instead of drying the ammonia, the sulfuric acid would destroy it.
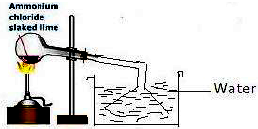
10) Explain with a diagram the preparation of aqueous ammonia.
Ans: Aqueous ammonia (ammonia water) is prepared by dissolving ammonia gas in water.
Procedure:
- Ammonia gas is generated in a round-bottom flask, typically by heating a mixture of an ammonium salt (like NH₄Cl) and a strong base (like Ca(OH)₂).
- The generated ammonia gas is passed through a drying tower containing quicklime (CaO) to remove any moisture. (Conc. H₂SO₄ cannot be used, as explained in Q9c).
- The dry ammonia gas is then led through an inverted funnel that dips into a beaker containing distilled water.
- Ammonia is highly soluble in water. The inverted funnel design prevents water from being “sucked back” into the hot flask due to the rapid dissolution of the gas. As the gas dissolves, pressure drops in the delivery tube, and water would rise, but it only rises to the rim of the wide funnel, breaking the seal and stopping the back-suction.
- The dissolution process forms ammonium hydroxide in water (NH₃ + H₂O → NH₄OH), which is the aqueous ammonia.
Diagram Description:
- A round-bottom flask on a stand with a burner underneath, labeled “Generator”.
- A glass delivery tube leading from the flask to a drying tower (U-tube or column) filled with lumps of “CaO”.
- Another delivery tube leading from the dryer to an inverted funnel.
- The inverted funnel is shown placed just below the surface of the water in a beaker labeled “Distilled Water”.
- The beaker is placed on a stand.