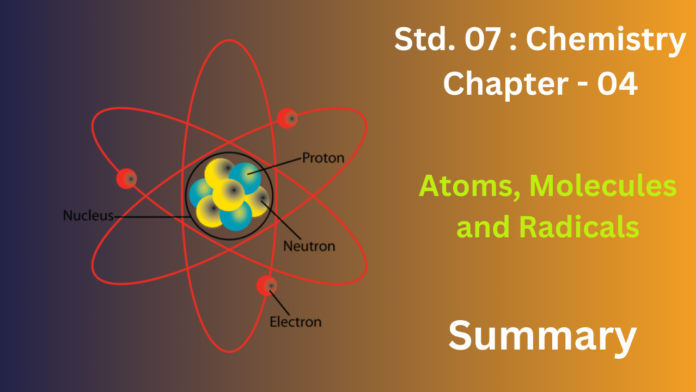
It likely starts by defining the atom as the smallest unit of an element that can take part in a chemical reaction. Key points might include:
- Atoms are made up of even smaller subatomic particles: protons (positive charge, in the nucleus), neutrons (no charge, in the nucleus), and electrons (negative charge, orbiting the nucleus).
- The nucleus is the central, dense part of the atom containing protons and neutrons.
- Electrons occupy specific energy levels or shells around the nucleus.
The chapter then introduces molecules as combinations of two or more atoms that are chemically bonded together. Key points might include:
- Molecules can be formed from atoms of the same element (e.g., O₂, N₂) or different elements (e.g., H₂O, CO₂).
- The chemical formula of a molecule shows the types and numbers of atoms present.
Finally, the chapter likely introduces the concept of radicals (or free radicals). Key points might include:
- Due to their unpaired electron, radicals are generally very reactive and unstable.
- Examples of radicals might be mentioned in simple contexts.
In short, the chapter lays the groundwork for understanding matter by explaining that it’s made of atoms, which combine to form molecules. It also introduces the idea of reactive species called radicals that have unpaired electrons. The focus is on basic definitions and understanding the composition of matter at a fundamental level.
EXERCISE
Question 1.
Define the following terms :
Answer:
- Atom : An atom represents the most fundamental and indivisible unit of a chemical element that can participate in a chemical reaction without being broken down into simpler substances by ordinary chemical means. Structurally, an atom comprises a central, positively charged nucleus, which contains protons (positively charged subatomic particles) and neutrons (electrically neutral subatomic particles). This nucleus accounts for the majority of the atom’s mass. Surrounding the nucleus is a cloud of negatively charged electrons that occupy specific energy levels or orbitals. The number of protons in the nucleus defines the element to which the atom belongs (this number is known as the atomic number). Atoms of the same element possess an identical number of protons.
- Molecule : A molecule constitutes a discrete entity formed by the chemical bonding of two or more atoms. These constituent atoms can be of the same chemical element, as seen in diatomic oxygen (O₂) or nitrogen (N₂), or they can be atoms of different chemical elements, as exemplified by water (H₂O) or carbon dioxide (CO₂). The chemical bonds that hold the atoms together within a molecule arise from the sharing or transfer of electrons between the atoms. A molecule represents the smallest unit of a chemical compound that retains the characteristic chemical properties of that specific compound. When a compound exists in its molecular form, a single molecule is representative of the entire substance in terms of its chemical behavior.
- Radicals : Radicals are atoms, molecules, or ions with one or more unpaired electrons, making them highly reactive.
- Valency : Valency is the measure of an atom’s capacity to form chemical bonds with other atoms. It is conceptually understood as the number of chemical bonds that an atom of a particular element can typically establish when forming chemical compounds or molecules. This combining power is often determined by the number of electrons in the atom’s outermost electron shell, known as valence electrons, which are available for sharing, gaining, or losing in order to achieve a stable electron configuration, often resembling that of a noble gas. The valency of an element indicates the number of univalent atoms (like hydrogen or chlorine) that one atom of that element can combine with.
Question 2.
Write the names of the elements present in the following compounds.
Ans:
(a) Water (H2O): Hydrogen, Oxygen
(b) Carbon Dioxide (CO2): Carbon, Oxygen
(c) Methane (CH4): Carbon, Hydrogen
(d) Sodium Chloride (NaCl): Sodium, Chlorine
Question 3.
What does each of the following represent ?
Ans:
- 2C02 = 2 molecules of carbon dioxide.
- 2H2S = 2 molecules of hydrogen sulphide.
- 5H2S04 = 5 molecules of sulphuric acid.
- 6NaNO3 = 6 molecules of sodium nitrate.
Question 4.
Write the symbols and valencies of the following radicals:
Ans:
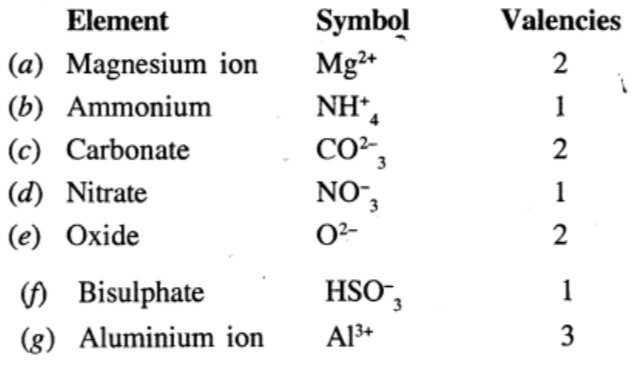
Question 5.
Name the following radicals :
Ans:
- SO42- = Sulphate
- HC03– = Bicarbonate
- OH- = Hydroxide
- Cr2072- = Dichromate
Question 6.
- Name one ion for each of the valencies +1, +2 and +3.
- Name one ion for each of the valencies-1, -2 and -3.
Ans:
Positive Valencies:
- +1: Sodium ion (Na+)
- +2: Magnesium ion (Mg2+)
- +3: Aluminum ion (Al3+)
Negative Valencies:
- -1: Chloride ion (Cl−)
- -2: Oxide ion (O2−)
- -3: Nitride ion (N3−)
Question 7.
The valency of calcium is 2. Write the valencies of other radical in the following :
- CaO
- Ca(OH)2
- CaC03
- CaCl2
Ans:
- O= 2
- OH = 1
- CO3 = 2
- Cl = l
Question 8.
Write the names of the following compounds :
Ans:

Question 9.
Write the molecular formulae of:
Ans:
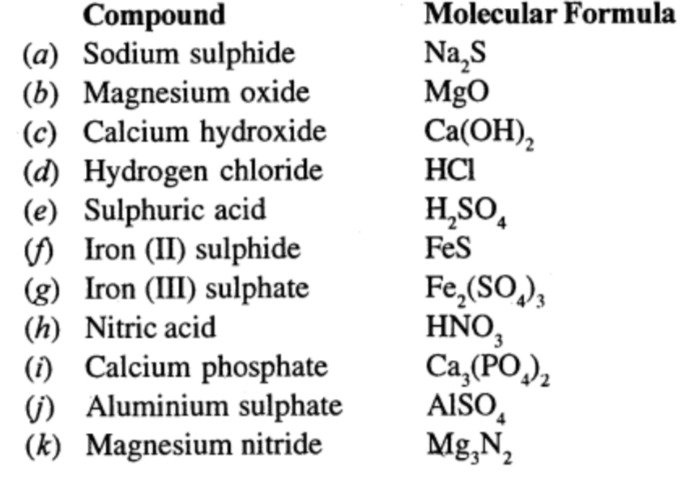
Question 10.
The valency of sodium is one, write the molecular formula for the following compounds of sodium.
- sodium oxide : Na20
- sodium sulphate : Na2S04
- sodium carbonate : Na2CO3
- sodium hydroxide : NaOH
- sodium nitrate : NaN03
Question 11.
What is variable valency ? Give two examples of elements showing variable valency.
Ans:
Variable valency: Some elements can have different combining capacities in different compounds.
Examples: Iron (+2, +3), Copper (+1, +2).
Question 12.
Give the group number of following elements present in periodic table
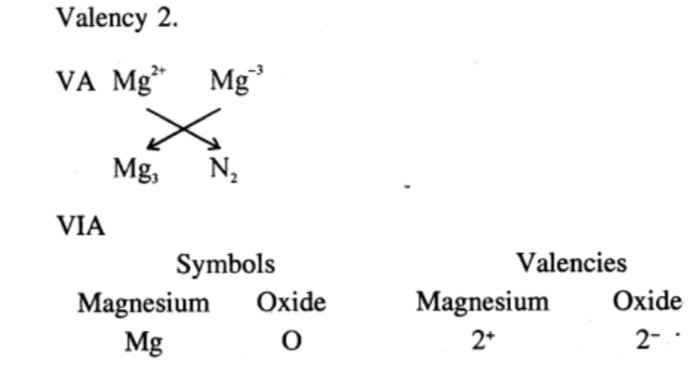
- Magnesium : IIA
- Carbon : IVA
- Sulphur : VIA
- Neon : Zero
Question 13.
An element belongs to group VA. What would be its valency? Name two such elements.
Ans:
An element residing in Group VA (also designated as Group 15) of the periodic table possesses a characteristic of five electrons in its outermost electron shell, which are known as valence electrons. To attain a stable electron configuration, typically an octet (eight electrons in the valence shell, resembling the noble gases), these elements exhibit a strong tendency to gain three additional electrons.
Consequently, the most prevalent valency displayed by elements belonging to Group VA is 3. This negative valency signifies their capacity to accept three electrons to complete their valence shell and form chemical bonds.
It is also noteworthy that under specific chemical conditions, particularly when interacting with highly electronegative elements such as oxygen or fluorine, some elements within Group VA can exhibit positive valencies. This occurs when they lose their valence electrons, resulting in oxidation states of +3 or +5. However, the -3 valency remains the most common and characteristic for the group as a whole.
Two prominent examples of elements that are members of Group VA are:
- Nitrogen (N)
- Phosphorus (P)
Question 14.
An element belongs to group II. What would be its valency? Write the formula of molcules of compounds it will form with elements in VA, VIA and VIIA groups.
Ans:
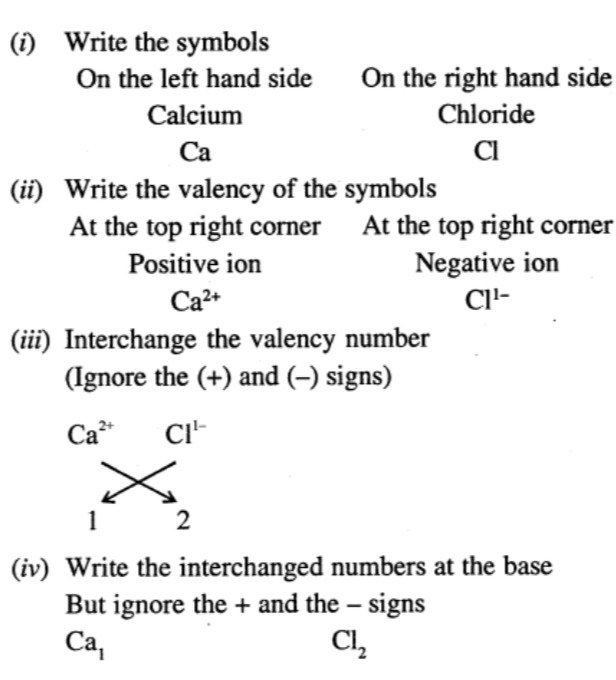
OBJECTIVE TYPE QUESTIONS
1. Fill in the blanks:
- Atoms are.
Ans : neutral
- An ion with positive charge is called————.
Ans : cation
- An ion with negative charge is called———-.
Ans : anion
- 2H2 means two ———- of hydrogen.
Ans : atoms
- ———— is a triatomic molecule.
Ans : Ozone
- Metals have ———— valency.
Ans : variable
- Chemical name of caustic soda is———-.
Ans : sodium hydroxide NaOH
2. Tick (√) the correct answer.
(a) The valency of iron in Fe203 is
- 1
- 2
- 3
- 6
(b) Which of the following has valency 4 ?
- aluminium
- oxygen
- carbon
- phosphorus
(c) The sulphate radical is written as S042-. What is the formula of calcium sulphate ?
- Ca(S04)2
- Ca2(S04)
- Ca(S04)3
- CaS04
(d) Which of the following exhibit variable valency ?
- calcium
- copper
- carbon
- chlorine
3. State the term for the following:
- The number of atoms present in a molecule of an element – atomicity.
- The symbolic representation of a molecule – molecular formula.
- A group of atoms that react as a single unit – molecule.
- The combining capacity of an element – valency.
- The tabular arrangement of elements in horizontal rows and vertical columns – periodic table.