1. Electric Current & Circuit
Electric Current: The continuous flow of tiny, negatively charged particles called electrons through a conductor.
Electric Circuit: The complete, closed path needed for current to flow. Its essential parts are:
A source (e.g., battery) to provide energy.
Conducting wires to carry the current.
A load (e.g., bulb) that uses the energy.
A switch to turn the circuit ON or OFF.
2. Circuit Types
Closed Circuit: The path is complete. Current flows, and the appliance works.
Open Circuit: The path is broken. Current cannot flow, and the appliance is off.
3. Conductors & Insulators
Conductors: Materials that allow current to flow easily (e.g., copper, iron, aluminum).
Insulators: Materials that do not allow current to flow (e.g., rubber, plastic, wood). Wires are coated with insulators for safety.
4. Circuit Diagrams
Simple, universal symbols are used to represent circuit components in a neat and clear diagram.
5. Heating Effect of Current
When current flows through a wire, it generates heat due to the resistance of the material.
Uses: Electric iron, heater, toaster.
Fuse: A safety device with a thin wire that melts and breaks the circuit if the current becomes too high, protecting appliances.
6. Magnetic Effect of Current
An electric current creates a magnetic field around it.
Electromagnet: A strong, temporary magnet made by passing current through a coil wound around a soft iron core.
Uses: Electric bells, cranes for lifting scrap metal, MRI machines.
7. Chemical Effect of Current
Electric current can cause chemical changes when passed through a liquid (conducting solution).
Uses:
Electroplating: Depositing a layer of one metal onto another to prevent rusting or for a shiny look (e.g., chromium on car parts, gold plating).
Refining of Metals: Purifying impure metals, like copper.
Test yourself
A. Objective Questions
1. Write true or false for each statement:
(a) A fuse wire has a high melting point.
Ans: False.
(b) Flow of protons constitutes electric current.
Ans: False.
(c) Silver is an insulator of electricity.
Ans: False.
(d) S.I. unit and commercial unit of electrical energy are same.
Ans: False.
(e) Overloading of electric current in circuits can lead to short circuiting.
Ans: True.
(f) Our body can pass electricity through it.
Ans: True.
(g) All metals are insulators of electricity.
Ans: False.
(h) The earth wire protects us from an electric shock.
Ans: True.
(i) A switch should not be touched with wet hands.
Ans: True.
(j) AH electrical appliances in a household circuit work at the same voltage.
Ans: True.
(k) In a cable, the green wire is the live wire.
Ans: False.
(l) A fuse is connected to the live wire.
Ans: True.
(m) A switch is connected to the neutral wire.
Ans: False.
2. Fill in the blanks
(a) The unit in which we pay the cost of electricity is kWh.
(b) The electrical energy consumed in a house is measured by kWh meter.
(c) In a household electrical circuit, the appliance are connected in parallel with the mains.
(d) A switch is connected to the live wire.
(e) The red colour insulated wire in a cable is the live wire.
(f) One kilowatt hour is equal to 3.6 x 106 joule.
(g) A fuse wire should have low melting point.
Ans:
(a) The unit in which we pay the cost of electricity is kWh.
(b) The electrical energy consumed in a house is measured by kWh meter.
(c) In a household electrical circuit, the appliance are connected in parallel with the mains.
(d) A switch is connected to the live wire.
(e) The red colour insulated wire in a cable is the live wire.
(f) One kilowatt hour is equal to 3.6 x 106 joule.
(g) A fuse wire should have low melting point.
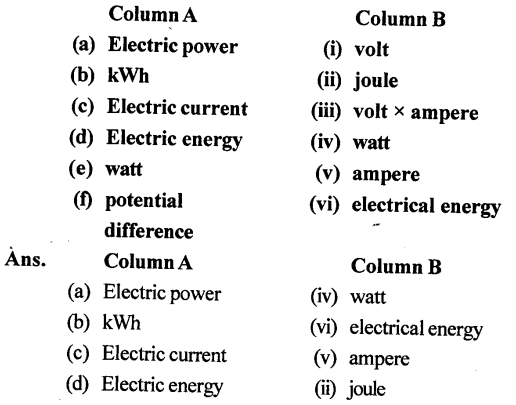
3. Match the following
4. Select the correct alternative
(a) All wires used in electric circuits should be covered with
- colouring material
- conducting material
- an insulating material
- none of the above
Ans: an insulating material
(b) Electric work done per unit time is
- electrical energy
- electric current
- electric voltage
- electrical power
Ans: electrical power
(c) One kilowatt ¡s equal to
- 100 watt
- 1000 watt
- 10 watt
- none of these
Ans: 1000 watt
(d) Fuse wire is an alloy of
- tin-lead
- copper-lead
- tin-copper
- Lead-silver
Ans: tin-lead
(e) A fuse wire should have
- a low melting point
- high melting point
- very high melting point
- none of the above
Ans: a low melting point
(f) When switch of an electric appliance is put off, it disconnects
- the live wire
- the neutral wire
- the earth wire
- the live and the neutral wire
Ans: the live wire
(g) The purpose of an electric meter in a house is
- to give the cost of electricity directly
- to give the consumption of electrical energy
- to safeguard the circuit from short circuiting
- to put on or off the mains.
Ans: to give the consumption of electrical energy
(h) If out of the two lighted bulbs in a room, one bulb suddenly fuses, then
- other bulb will glow more
- other bulb will glow less
- other bulb will also fuse
- other bulb will remain lighted unaffected.
Ans: other bulb will remain lighted unaffected.
B. Short/Long Answer Questions
Question 1. What do you understand by electricity at rest ?
Ans: That tiny zap from a doorknob or a balloon sticking to a wall is static electricity. It’s often called “electricity at rest” because the charge builds up and stays on a surface until it can escape.This happens when two objects rub together, like your shoes on a carpet. Electrons rub off one material and onto the other. The object that gains electrons becomes negatively charged, while the one that loses them becomes positively charged.These stationary charges create invisible forces. Opposites attract, so your positively charged hair strands repel each other and stand on end. A negatively charged balloon is attracted to a neutral wall. The charge waits until it slowly leaks away or escapes in a sudden zap.
Question 2. Why does a plastic comb rubbed with dry hair attract bits of paper ? Ans: When a plastic comb is rubbed with dry hair, it gains an electric charge through friction.Rubbing transfers electrons from the hair to the comb, making the comb negatively charged.When this charged comb is brought near tiny bits of paper (which are neutral), it induces a positive charge on the near side of the paper.Since unlike charges attract, the comb pulls the paper bits toward it.
Question 3. Who discovered the way of producing electricity by friction?
Ans: The discovery that electricity could be produced by friction is not credited to a single individual in a single moment, but rather it was a gradual process of observation and experimentation that began in ancient times.
The earliest known observations come from Ancient Greece, around 600 BCE. The philosopher Thales of Miletus noted that when a piece of amber (a fossilized tree resin known in Greek as ‘ēlektron’) was rubbed with animal fur, it gained the mysterious ability to attract lightweight objects like feathers or bits of straw. This was a static electrical charge generated through friction. However, Thales and the Greeks did not understand this as “electricity”; they saw it as a unique property of amber itself.
For centuries, this phenomenon remained a curious novelty. The real progress began during the Renaissance and the Scientific Revolution. In the 16th century, English scientist William Gilbert conducted a systematic study of this effect. He tested many other materials besides amber and found that several of them could be electrified by friction. He coined the term “electricus” (from the Greek word for amber) to describe materials that exhibited this property, which is how the word “electricity” eventually originated. Gilbert was the first to rigorously demonstrate that friction could produce an electrical effect in a variety of substances.
Later, in the 17th and 18th centuries, other scientists like Otto von Guericke (who built the first static electricity generator), Stephen Gray (who discovered electrical conduction), and Charles François de Cisternay DuFay (who identified two types of electricity) built upon this foundational knowledge. Their collective work, all stemming from the initial principle of generating electricity by friction, ultimately paved the way for Benjamin Franklin’s famous experiments and our modern understanding of electrical science.
So, while Thales made the initial observation, it was William Gilbert who is most credited with discovering and scientifically establishing the method of producing electricity by friction.
Question 4. Name two substances which canbe charged by friction.
Ans: Two common examples of charging by friction are:
Amber: When rubbed with wool, amber becomes negatively charged as it gains electrons from the wool.
Glass: When rubbed with silk, glass becomes positively charged as it loses electrons to the silk.
Question 5. What are the two kinds of charges ?
Ans: The names “positive” and “negative” for electric charges originated with Benjamin Franklin. He arbitrarily labeled the charge on a glass rod rubbed with silk as “positive.” The charge on plastic rubbed with wool became “negative.”
Their fundamental behavior is simple: like charges repel, and opposite charges attract. So, positive repels positive, negative repels negative, but positive and negative pull towards each other.
We now know positive charge comes from protons in an atom’s nucleus, and negative charge comes from the electrons orbiting it. The interaction between these opposite charges is the basis for all electrical phenomena.
Question 6. A glass rod is rubbed with silk. State the kind of charge acquired by each.
Ans:
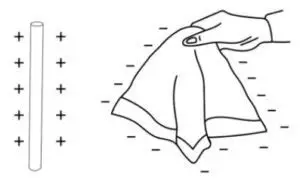
When a glass rod is rubbed with a piece of silk, a transfer of electrons occurs between the two materials due to the triboelectric effect.
The glass rod loses some of its electrons to the silk. Since electrons are negatively charged, the loss of negative charge leaves the glass rod with a net positive charge.
The silk cloth gains the electrons that were lost by the glass rod. This excess of electrons gives the silk a net negative charge.
Question 7. An ebonite rod is rubbed with fur. State the kind of-charge acquired by each.
Ans: When an ebonite rod is rubbed with fur, electrons are transferred between the two objects.
The ebonite rod acquires a negative charge.
The fur acquires a positive charge.
This happens because during the rubbing process, electrons from the fur are transferred to the ebonite rod. The ebonite rod, having gained extra electrons, becomes negatively charged. Conversely, the fur, having lost electrons, is left with a net positive charge.
Question 8. Describe an experiment to demonstrate that there are two r kinds of charges.
Ans: Aim: To show that there are two, and only two, types of electric charges—which we call positive and negative—and to demonstrate their interaction.
Principle: The experiment is based on a very simple rule: Like charges repel each other, while unlike charges attract each other. By creating charged objects and observing how they interact, we can identify the nature of their charges.
Materials Required:
Two inflated balloons with strings attached.
A glass rod.
A silk cloth (e.g., a silk scarf).
A plastic comb or a rubber rod.
A woolen cloth or fur.
A stand to suspend a balloon (optional).
Procedure and Observations:
Step 1: Creating Charged Objects
We first need to charge two objects in the same way to see if they behave similarly.
Take the first balloon and rub it vigorously against your hair or the woolen cloth. Due to friction, the balloon becomes electrically charged.
Suspend this charged balloon from a stand using a string so that it is free to move and rotate. It is now our sensitive “charge detector.”
Now, take the second balloon and rub it similarly against your hair or wool. This second balloon has been charged in the same way as the first.
Step 2: Observing Interaction Between Similarly Charged Objects
Slowly bring the second charged balloon close to the suspended first balloon.
Observation: You will see that the suspended balloon moves away from the balloon in your hand. It is clearly repelled.
Conclusion from Step 2: This repulsion is crucial. It proves that the two objects possess the same kind of charge. According to our principle, like charges repel. We can call this charge, for example, Negative charge (which is accurate, as rubbing with hair or wool gives a negative charge).
Step 3: Creating a Different Kind of Charge
Now, we need to create a different type of charge to see a different interaction.
Take the glass rod and rub it thoroughly with the silk cloth. This process charges the glass rod.
The suspended balloon is still negatively charged from Step 1.
Step 4: Observing Interaction Between Differently Charged Objects
Bring the charged glass rod close to the negatively charged suspended balloon.
Observation: This time, you will see that the balloon moves towards the glass rod. It is clearly attracted.
Conclusion from Step 4: This attraction proves that the charge on the glass rod is different from the charge on the balloon. Since the balloon is negative, the glass rod must have the opposite charge, which we call Positive charge.
Question 9. How will you show that like charges repel and unlike charges attract each other ?
Ans: To show that like charges repel and unlike charges attract, you can perform a simple experiment using two suspended charged objects.
Suspend a charged glass rod (positively charged) using a thread.
Bring another positively charged glass rod near it. The suspended rod will move away, showing that like charges repel.
Now, bring a charged ebonite rod (negatively charged) near the suspended positively charged glass rod. The suspended rod will move towards it, showing that unlike charges attract.
This demonstrates the fundamental rule of electrostatics: Like charges repel, unlike charges attract.
Question 10. A glass rod rubbed with silk is suspended near an ebonite r rod rubbed with fur. What will be your observation ? Give a reason to your answer.
Ans: Observation:The glass rod and ebonite rod will attract each other.
Reason:
Rubbing the glass rod with silk makes it positively charged, while rubbing the ebonite rod with fur makes it negatively charged. Unlike charges attract each other, so they move closer when brought near.
Question 11. An ebonite rod rubbed with fur is suspended near another ebonite rod rubbed with fur. State your observation and give a reason to support your answer.
Ans: Observation: The two ebonite rods will repel each other.
Reason: When ebonite rods are rubbed with fur, both gain the same type of negative charge. Since like charges repel, the two negatively charged rods push each other away.
Question 12. What do you mean by conservation of charges ?
Ans: Conservation of Charge
This is a fundamental rule of nature. It means the total electric charge in a system never changes.
In simpler words:
You cannot create or destroy net charge.
Charge can only move from one object to another.
A simple example:
When you rub a balloon on your hair, electrons (which are negative) rub off from your hair onto the balloon.
The balloon gains negative charge.
Your hair loses negative charge, making it positive.
No charge was created or destroyed. The total amount of charge before and after rubbing is exactly the same. The charges were just separated.
Question 13. An ebonite rod is rubbed with fur. Compare the charges acquired by them.
Ans: When an ebonite rod is rubbed with fur, the ebonite rod acquires a negative charge, and the fur acquires a positive charge.The charges are equal in magnitude but opposite in nature. This happens because electrons are transferred from the fur to the ebonite rod during rubbing.
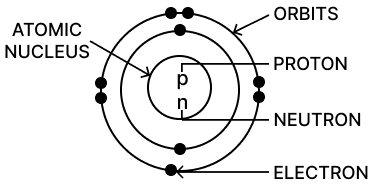
Question 14. Name three constituents of an atom and state the kind of charge on each of them.
Ans: The three fundamental constituents of an atom are protons, neutrons, and electrons.
Proton: A proton is a positively charged particle. It is found in the central core, or nucleus, of the atom. The number of protons in an atom determines which chemical element it is.
Neutron: A neutron is a neutral particle, meaning it has no electrical charge (it is neither positive nor negative).
Electron:An electron is a negatively charged particle. These particles are extremely light and revolve rapidly around the nucleus in regions often called electron shells or orbitals.
Question 15. What is the net charge on an atom ?
Ans: An atom has a net charge of zero because it is electrically neutral. This occurs when the number of positively charged protons in the nucleus is equal to the number of negatively charged electrons surrounding it. Their equal but opposite charges cancel each other out, resulting in no overall charge.
Question 16. Briefly describe the structure of an atom.
Ans: An atom has a net charge of zero because it is electrically neutral. This occurs when the number of positively charged protons in the nucleus is equal to the number of negatively charged electrons surrounding it. Their equal but opposite charges cancel each other out, resulting in no overall charge.
Question 17. What are free electrons ?
Ans: Free electrons are not bound to any atom. This lets them move easily, particularly in metals. Applying a voltage causes these electrons to flow, creating an electric current.
Question 18. What causes the charging of two objects when they are rubbed together ?
Ans:
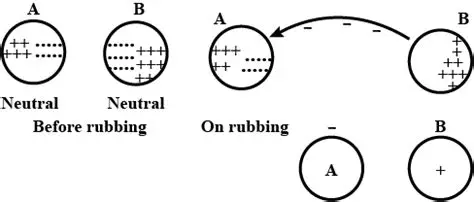
When two objects rub together, electrons move between them.
This occurs because some materials hold their electrons more tightly than others.
The material with a stronger hold pulls electrons from the material with a weaker hold.
The object that gains electrons becomes negatively charged.
The object that loses electrons becomes positively charged.
This process is called charging by friction.
Question 19. In each of the following cases, state which body loses electrons: (a) A glass rod when rubbed with silk. (b) An ebonite rod when rubbed with fur.
Ans: (a) A glass rod when rubbed with silk:
The friction between the two materials causes electrons to transfer from the glass onto the silk cloth. As a result, the glass rod becomes positively charged (due to a deficit of electrons), and the silk becomes negatively charged (due to an excess of electrons).
(b) An ebonite rod when rubbed with fur:
When an ebonite rod is rubbed with fur, the fur loses electrons. In this case, electrons are transferred from the fur to the ebonite rod. Consequently, the ebonite rod gains electrons and becomes negatively charged, while the fur, having lost electrons, becomes positively charged.
Question 20. A glass rod is rubbed with silk. Explain the charging of the glass rod and the silk on the basis of electron movement.
Ans: When a glass rod is rubbed vigorously with a silk cloth, both objects become electrically charged, but with opposite types of charge.
Here is a step-by-step explanation based on electron movement:
Initial State: In their neutral states, both the glass rod and the silk cloth have an equal number of positive protons and negative electrons. Therefore, their net charge is zero.
The Rubbing Process: The act of rubbing brings the glass and silk into very close contact. Different materials have different affinities, or “hold,” on their electrons. In this specific case, the atoms in the silk cloth have a stronger attraction for electrons than the atoms in the glass rod.
Electron Transfer: Due to this difference in electron affinity, when rubbed together, some of the loosely held electrons from the atoms on the surface of the glass rod are transferred to the silk cloth.
Final Charged State:
The Glass Rod: After losing electrons, the glass rod now has more protons (positive charges) than electrons (negative charges). As a result, it acquires a net positive charge.
The Silk Cloth: The silk cloth, having gained the extra electrons, now has more electrons (negative charges) than protons (positive charges). As a result, it acquires a net negative charge.
Question 21. An ebonite rod is rubbed with fur. Explain the charging of the ebonite rod and the fur on the basis of electron movement.
Ans: When the ebonite rod is rubbed with fur, electrons are transferred from the fur to the rod.
This happens because the fur has a weaker hold on its electrons compared to the ebonite. As a result, the ebonite rod gains extra electrons and becomes negatively charged. Conversely, the fur loses electrons and becomes positively charged.
Question 22. Distinguish between conductors and insulators of electricity.
Ans: Conductors
Function: They allow electric current to flow through them easily.
Reason: They have free electrons which can move freely when a voltage is applied.
Examples: Metals like copper, silver, aluminum, and iron.
Insulators
Function: They do not allow electric current to flow through them easily.
Reason: Their electrons are tightly bound to their atoms and are not free to move.
Examples: Rubber, plastic, wood, glass, and dry air.
In short: Conductors are like a wide-open path for electricity, while insulators are like a strong barrier that blocks it.
23.Give one example each of a conductor and an insulator of electricity
Ans: Conductor example: Copper
Insulator example: Rubber
Question 24. State two ways of charging a conductor.
Ans:
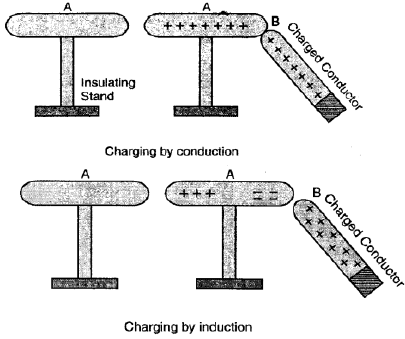
Two ways of charging a conductor are:
Charging by Conduction (or Contact):
When a charged object is brought into direct contact with a conductor, charge (either positive or negative) is transferred from one to the other, charging the conductor.
Charging by Induction:
When a charged object is brought close to, but without touching, a conductor, it causes a redistribution of the conductor’s charges. If the conductor is then earthed (grounded) momentarily, it retains a charge opposite to that of the inducing charged object.
Question 25. Name the way of charging a conductor in which the charge is shared.
Ans: The method of charging a conductor in which the charge is shared between two objects is called charging by conduction (also commonly known as charging by contact).
Explanation:
In this process, a charged object is brought into direct physical contact with an uncharged conductor. Upon contact, the electric charge (either positive or negative) redistributes itself across the surfaces of both objects because charges are free to move within conductors. The objects share the total charge until they both reach the same electric potential. As a result, the originally uncharged conductor acquires a net charge that has the same sign as the charging object.
Question 26. Describe the method of charging a conductor by conduction.
Ans:
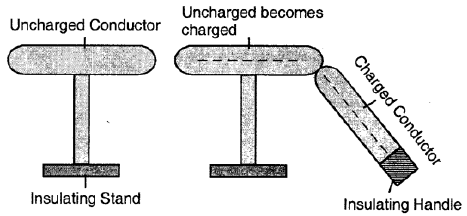
During charging by conduction:
A charged object touches a neutral conductor.
Electrons transfer between them.
The conductor gains the same type of charge as the charged object.
After sharing charge, both objects repel each other.
Question 27. A metal rod A is to be charged positively by using another charged rod B. What should be the kind of charge on the rod B if charging is to be done by conduction ?
Ans: This transfer leaves Rod A with fewer electrons, so it becomes positively charged.
After separating, both rods carry a positive charge.
Question 28. Explain the charging by conduction in terms of movement of electrons.
Ans: When a negatively charged object (like a plastic rod rubbed with wool) is touched to a neutral object, electrons are repelled by the excess electrons on the rod.These repelled electrons move away from the point of contact through the neutral object, leaving that area positively charged.When the objects are separated, the neutral object is now left with an overall positive charge. It has been charged by conduction, acquiring the same type of charge as the rod originally had.
Question 29. Describe the method of charging a conductor by induction.
Ans: Charging a conductor by induction is a method where a conductor is charged without any physical contact with the charging body. It uses the redistribution of charges in the conductor due to the presence of a nearby charged object.
Steps involved:
Bring a charged object (e.g., a negatively charged rod) close to an uncharged conductor without touching it.
Charges in the conductor redistribute — unlike charges are attracted toward the near end, and like charges are repelled to the far end.
Ground the far end of the conductor (touching with a wire or hand), allowing the repelled like charges to flow to the earth.
Remove the grounding connection first, then remove the charged rod.
The conductor is now left with a net charge opposite to that of the charging object.
This method produces a charge on the conductor opposite to the inducing charge.
Question 30. Explain the charging by induction in terms of movement of electrons.
Ans:
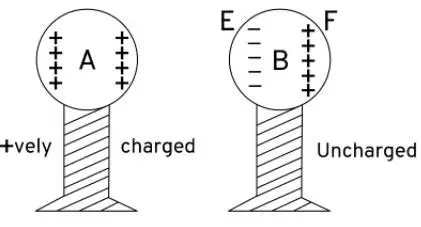
Charging by Induction (using movement of electrons):
Bring a charged object near a neutral conductor (e.g., a negatively charged rod near a metal sphere).These electrons move to the far side of the conductor.
This movement creates a charge separation:
The side near the rod now has a positive charge (loss of electrons).The side far from the rod now has a negative charge (excess electrons).Ground the conductor on the far side (the negatively charged side).The repelled electrons are pushed out of the conductor and into the ground.Remove the ground connection first, then remove the charged rod.The conductor is now left with a net positive charge, because it has lost electrons.In short: Electrons are repelled and flow away, leaving the object with an opposite charge to the inducing charge, without any physical contact.
Question 31. Figure below shows a metal rod AB placed on an insulating stand. In figure (a) a negatively charged ebonite rod C is touched w ith the metal rod AB, while in figure (b), the negatively charged ebonite rod C is held near the rod AB. State the kind of charges at the ends A and B of the rod, in each case.
Ans:
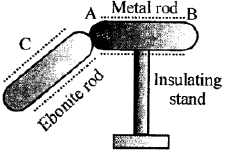
Case (a): When the negatively charged ebonite rod C is touched with metal rod AB:
Electrons transfer from C to AB.
A: Negative charge
B: Negative charge
Case (b): When the negatively charged ebonite rod C is held near metal rod AB (without touching):
By induction:
End A (near C): Positive charge
End B (far from C): Negative charge
Question 32. Can you charge an insulator by thg method of conduction ?
Ans:In conduction, charge is transferred through direct contact. Since insulators have no free electrons to move through their entire structure, the charge remains only at the point of contact and does not spread.
Question 33. What is an electroscope ? Name the two types of electroscopes.
Ans:
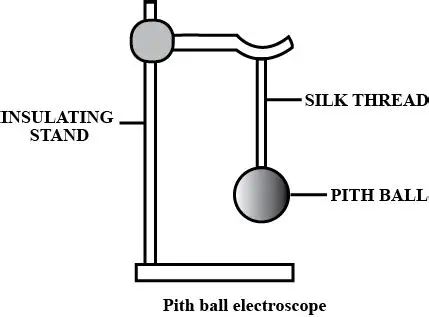
An electroscope is a simple scientific instrument used to detect the presence of an electric charge on a body. It works on the principle of like charges repel each other. When a charged object is brought near or in contact with the electroscope, it causes its internal components to gain a similar charge. Since these components now have the same type of charge, they repel each other and move apart. This movement provides a visual indication that an electric charge is present.
The two main types of electroscopes are:
The Gold-Leaf Electroscope: It consists of a metal rod with a metal disc or knob at the top, which is attached to two thin, flexible leaves of gold foil. The leaves are housed inside a glass jar to protect them from air currents. When charged, the two gold leaves gain the same charge and repel each other, standing apart at an angle. The amount of divergence (how far they spread apart) gives a rough idea of the amount of charge present.
The Pith-Ball Electroscope: This is a simpler version. It is made of a small, lightweight ball made from the pith of a plant (or a similar lightweight material like styrofoam), suspended by a non-conducting thread. When a charged object is brought near the pith ball, it gets attracted initially (due to induction) and then, upon contact, gets repelled if it acquires the same charge. The movement of the pith ball away from the object indicates the presence of a charge
Question 34. Describe a pith ball electroscope. how can you use it to test whether a body is charged or uncharged ?
Ans:
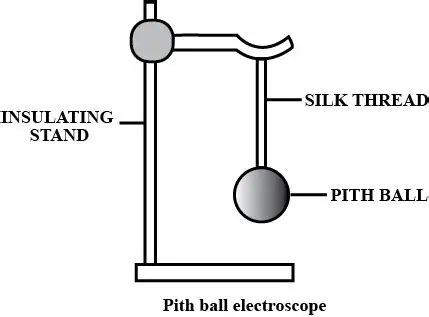
It consists of one or two small, lightweight balls made from the pith of a plant (or similar light material like styrofoam), suspended by a non-conducting thread from a stand.
How to Test if a Body is Charged or Uncharged
To test an object:
First, bring the object close to the uncharged pith ball without touching it.
Observation and Conclusion:
If the pith ball is attracted to the object, then the object is charged. This initial attraction occurs because the charge on the object induces an opposite charge on the near side of the pith ball, causing attraction.This simple test relies on the principle of electrostatic induction to detect the presence of charge.
Question 35. How will you use a pith ball electroscope to find out whether the charge on a charged body is positive or negative ?
Ans: Aim: To determine the nature (positive or negative) of charge on a charged body using a pith ball electroscope.
Apparatus: Pith ball electroscope, charged body (whose charge is to be identified), and a known material (like glass rod rubbed with silk, which is positively charged).
Procedure:
First, charge the pith ball electroscope by touching it with a body known to have a positive charge (e.g., a glass rod rubbed with silk). The pith ball will become positively charged and will be repelled by the glass rod.
Now, bring the unknown charged body close to this positively charged pith ball.
Observation and Inference:
If the pith ball moves away (is repelled) by the unknown body, then the charge on the body is positive.
If the pith ball moves towards (is attracted) to the unknown body, then the charge on the body is negative. This is because unlike charges attract each other.
Conclusion: By observing the nature of the force (attraction or repulsion) between the pith ball and the unknown charged body, we can identify the type of charge on the body.
Question 36. Draw a labelled diagram of a gold leaf electroscope and describe its construction.
Ans:
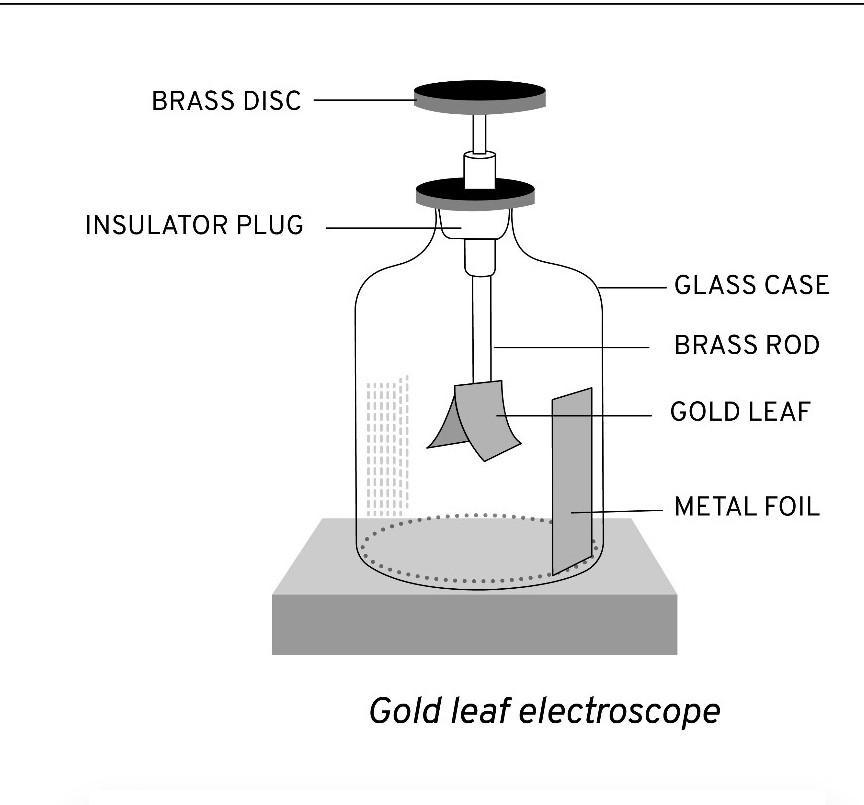
Labelled Diagram of a Gold Leaf Electroscope
[A simple hand-drawn diagram would show the following parts:]
Metal Cap: A circular brass disc at the very top.
Metal Rod: A brass rod connected to the cap, passing vertically down through the top.
Glass Case: A glass enclosure that houses the lower parts, mounted on a wooden base.
Gold Leaves: Two very thin, lightweight leaves of gold foil attached to the bottom of the metal rod.
Brass Disc: A small disc or plate at the end of the rod inside the case (sometimes present to support the leaves when not charged).
Wooden Base: The foundation supporting the entire apparatus.
Front Glass Plate: (Often included as a label) The front side of the glass case, which can be opened for resetting the leaves.
Construction
A gold leaf electroscope is constructed to detect electric charge. Its main parts are:
It has a metal cap (usually brass) on top, which is connected to a metal rod that passes through an insulating plug (like cork) into a glass case.
Attached to the bottom end of this metal rod inside the case are two very thin, lightweight gold leaves.
The entire assembly is enclosed in a glass case to protect the delicate gold leaves from air currents. The case is mounted on a wooden base.
The key principle is that the metal cap, rod, and gold leaves are all electrically connected, while the insulating plug separates them from the glass case and the ground.
Question 37. A positively charged glass rod is touched with the disc of an uncharged gold leaf electroscope. What will be your observation?
Ans: When the positively charged glass rod touches the disc of the uncharged electroscope, the leaves will diverge (move apart).This happens because the positive charge from the rod is transferred to the electroscope by conduction. The metal disc and the two gold leaves become positively charged. Since like charges repel, the two positively charged leaves repel each other, causing them to diverge.
Question 38. How will you use a gold leaf electroscope to find out whether a body is charged or uncharged ?
Ans:
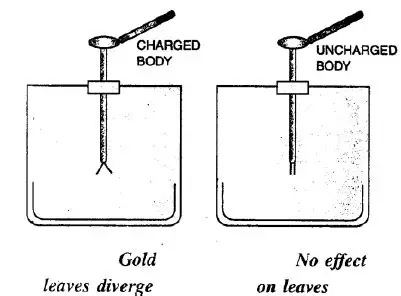
Charge the Electroscope: First, give the electroscope a known charge (say, positive) by touching its brass plate with a charged glass rod. The gold leaf will diverge (stand away) from the metal stem.
Bring the Test Body Near: Now, without touching, bring the test body close to the brass plate of the charged electroscope.
Observe the Leaf:
If the gold leaf diverges more, it means the test body has a similar charge (positive in this case).
If the gold leaf collapses (falls back) towards the stem, it means the test body has an opposite charge (negative in this case).
If there is no change in the leaf’s divergence, the test body is uncharged (neutral).
Question 39. How will you use a gold leaf electroscope to find out whether the charge on a charged body is positive or negative ?
Ans:
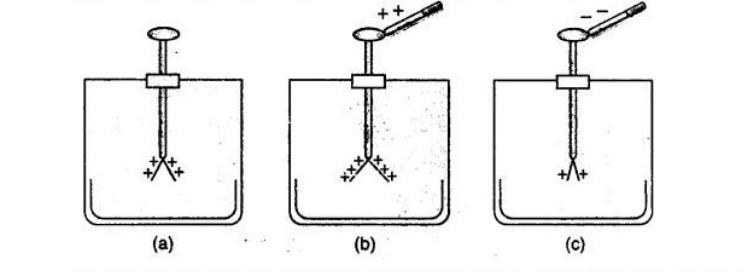
Step 1: Give a known charge to the gold leaf electroscope. For example, touch the brass cap with a glass rod rubbed with silk (giving the electroscope a positive charge). The gold leaf will stay diverged.
Step 2: Bring the charged body (whose charge is to be identified) near the brass cap of the positively charged electroscope. Do not let it touch.
Step 3: Observe the deflection of the gold leaf:
If the divergence of the leaf increases, the charge on the body is positive.
Reason: Like charges repel. The positive charge on the body repels more positive charge from the cap to the leaf, increasing divergence.
If the divergence of the leaf decreases, the charge on the body is negative.
Reason: Unlike charges attract. The negative charge on the body attracts positive charge from the leaf to the cap, reducing the leaf’s divergence.
Question 40. A negatively charged ebonite rod is touched with the disc of a negatively charged gold leaf electroscope. What will be your observation ?
Ans: When a negatively charged ebonite rod touches the disc of a negatively charged electroscope, the leaves collapse.This occurs because both objects have the same negative charge. The excess electrons on the electroscope’s leaves repel each other and flow away from the rod, moving through the person holding it (to the ground). This loss of charge neutralizes the electroscope, causing the leaves to fall.
Question 41. When a charged rod is touched with the disc of a positively charged gold leaf electroscope, it is observed that the divergence of leaves decreases. What is the kind of charge on the rod ?
Ans: The leaves collapse because the rod has a negative charge. The negative electrons from the rod neutralize the positive charge on the electroscope, reducing the repulsion between the leaves.
Question 42. Describe Franklin’s experiment. What did he conclude from his experiment ?
Ans: The Goal: To prove that lightning is a form of electricity.
The Experiment: During a thunderstorm in 1752, Franklin flew a kite with a metal wire attached. The hemp string was conductive, especially when wet, and had a metal key tied to the end. By insulating himself with a silk ribbon, he was able to bring his knuckle close to the key and draw a spark.
The Conclusions & Impact:
Franklin proved that lightning is a massive electrical spark, identical to the static electricity created in a lab.
This understanding led directly to his invention of the lightning rod. This device uses a grounded metal rod to safely divert lightning strikes away from buildings, preventing fires and saving countless lives.
Question 43. What causes lightning ?
Ans: Lightning is caused by the buildup and discharge of electrical energy in thunderstorms.
Inside a storm cloud, rising and falling air currents cause ice crystals and water droplets to collide. These collisions separate electrical charges, creating a positive charge near the top of the cloud and a negative charge near the bottom.
This strong negative charge in the cloud base repels electrons on the ground, making the ground positively charged. The air acts as an insulator, preventing the flow of electricity initially.
When the electrical charge difference becomes too great, the air can no longer resist. A giant, visible spark—lightning—occurs, either between different parts of a cloud, between two clouds, or between the cloud and the ground, as the electricity rapidly equalizes the charge imbalance.
Question 44. What are the effects of lightning ?
Ans: Effects of Lightning:
Destructive Effects:
Fire: Can instantly ignite trees, wooden structures, and houses.
Property Damage: Can destroy electrical appliances, disrupt power lines, and damage buildings.
Injury or Death: The massive electric current can cause severe burns, cardiac arrest, or direct fatal injury to humans and animals.
Beneficial Effects:
Fertilizes Soil: Lightning’s heat fixes atmospheric nitrogen into nitrates, which fall to earth with rain and act as natural fertilizer for plants.
Promotes Plant Growth: This added nitrogen helps in the growth of vegetation.
Cleans the Atmosphere: It helps remove certain greenhouse gases like methane from the air.
Question 45. What is a lightning conductor ? How does it work ?
Ans:
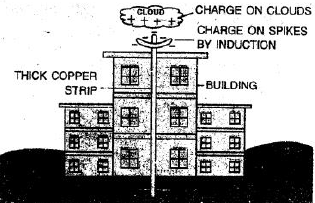
What is a Lightning Conductor?
A lightning conductor (or lightning rod) is a simple safety device used to protect tall buildings and structures from lightning strikes. It is a long, thick metal rod, usually made of copper, installed at the highest point of a building and connected to the ground with a low-resistance wire.
How Does it Work?
A lightning conductor works on the principle of providing a safe and easy path for the lightning’s electric charge to travel directly into the ground, without causing damage.
Here’s the process:
Attraction: The sharp, pointed metal rod attracts the incoming lightning strike because it is the easiest path for the electric charge.
Conduction: When lightning strikes the rod, the massive electrical current is captured.
Grounding: This dangerous current is then safely carried down the thick metal wire (called a down conductor) attached to the rod.
Dissipation: The wire leads to a large metal plate buried deep in the ground (called an earth plate), where the enormous electrical charge is safely dispersed into the earth.
Question 46. Mow is a tall building protected from damage due to lightning?
Ans:
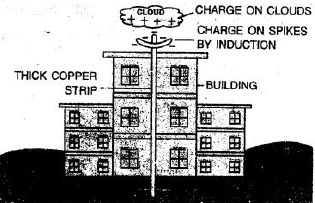
A tall building is protected from lightning damage by using a lightning conductor (also called a lightning rod). This system has three main parts:
Air Terminals (Rods): These are metal rods, usually made of copper or aluminum, placed at the highest points of the building (like the roof and spires).
Down Conductor: This is a thick metal cable that runs from the rods down the side of the building.
Grounding Electrode: This is a metal plate buried deep in the ground.
How it works:
The lightning rod does not attract lightning but provides a safe, easy path for the electric current to follow if lightning does strike. Instead of passing through the building’s structure and causing fire or explosion, the massive electrical charge is directed through the down conductor and safely dissipated into the ground. This protects the building and its occupants from damage.
Question 47. State three safety measures that you will observe in thunder storm.
Ans: During a thunderstorm, the primary danger is being struck by lightning. Here are three key safety measures to follow:
Seek Immediate Shelter in a Substantial Building: The safest place to be is inside a fully enclosed, substantial building with wiring and plumbing, such as a house, school, or shopping center. Once inside, avoid using corded electrical appliances, taking a shower, or washing dishes, as lightning can travel through a building’s wiring and metal pipes.
If Outdoors, Avoid Open Areas and Tall, Isolated Objects: If you cannot reach a safe building, never shelter under a lone tree, a gazebo, or in an open field. Lightning tends to strike the tallest object. Crouch down in a low-lying area, like a ravine or valley, but be mindful of potential flooding. Minimize your contact with the ground by crouching on the balls of your feet to reduce the risk from a ground current.
Stay Inside a Closed Vehicle as a Last Resort: If you are caught in your car, stay inside. A hard-topped vehicle (not a convertible or golf cart) can provide a degree of safety because if struck, the metal frame conducts the electrical charge around the outside of the vehicle and into the ground. Do not touch any metal surfaces inside the car during the storm.