This chapter primarily covers the preparation, properties, and uses of Hydrogen Chloride gas and its aqueous solution, Hydrochloric Acid.
Part 1: Preparation of Hydrogen Chloride Gas
Hydrogen Chloride gas is prepared in the laboratory by the reaction of common salt (Sodium Chloride) with concentrated Sulphuric Acid.
1. Laboratory Method:
Reactants: Sodium Chloride (NaCl) and Concentrated Sulphuric Acid (H₂SO₄).
Procedure: The reaction is carried out in a glass apparatus at room temperature or with mild warming.
Chemical Reaction:
NaCl + H₂SO₄ → NaHSO₄ + HCl
(This reaction occurs at room temperature or with mild warming)
Why Concentrated Sulphuric Acid? It is used because it is a non-volatile acid, which allows it to displace the more volatile HCl gas from its salt.
2. Collection: Since HCl gas is heavier than air and highly soluble in water, it is collected by downward displacement of air.
3. Drying: The moist gas can be dried by passing it through Concentrated Sulphuric Acid. It cannot be dried using basic drying agents like quicklime (CaO) or fused calcium chloride (CaCl₂) as they react with HCl.
Part 2: Properties of Hydrogen Chloride Gas
A. Physical Properties:
It is heavier than air.
It is extremely soluble in water. This is dramatically shown by the Fountain Experiment.
B. Chemical Properties:
Acidic Nature: Its aqueous solution is acidic and turns blue litmus red.
Reaction with Ammonia (NH₃): It reacts with ammonia gas to form dense white fumes of Ammonium Chloride (NH₄Cl). This is a test for HCl.
NH₃ + HCl → NH₄Cl
It is not a Reducing Agent: Unlike H₂S, HCl gas is not a reducing agent. It does not reduce acidified potassium permanganate or iron(III) salts.
Aqua Regia: When three parts of concentrated HCl and one part of concentrated HNO₃ are mixed, it forms Aqua Regia. This mixture can dissolve even gold and platinum.
Part 3: Hydrochloric Acid (Aqueous Solution of HCl)
When HCl gas is dissolved in water, it forms Hydrochloric Acid.
A. Preparation & Fountain Experiment:
The high solubility of HCl in water is proven by the Fountain Experiment. In this experiment, the dissolution of gas creates a strong fountain of water (turned pink by litmus) due to a drastic reduction in pressure inside the flask.
B. Chemical Properties of Hydrochloric Acid:
As a strong acid, it shows typical acid reactions:
Reaction with Metals: It reacts with metals above hydrogen in the reactivity series to form metal chlorides and hydrogen gas.
Zn + 2HCl → ZnCl₂ + H₂
Reaction with Metal Carbonates and Hydrogen Carbonates: It produces carbon dioxide gas.
Reaction with Metal Oxides and Hydroxides (Bases): It forms salt and water (neutralization reaction).
NaOH + HCl → NaCl + H₂O
Reaction with Salts (Precipitation Reactions):
It gives a white precipitate of Silver Chloride (AgCl) with Silver Nitrate solution. This precipitate is insoluble in nitric acid but soluble in ammonium hydroxide, forming a complex salt.
AgNO₃ + HCl → AgCl (↓) + HNO₃
(This is the standard test for Chloride ions)
It reacts with lead nitrate to form a white precipitate of lead chloride.
Part 4: Uses of Hydrochloric Acid
In Laboratories: As an important reagent.
In Industry:
For the manufacture of dyes, paints, and pigments.
For the preparation of chlorides and glues.
In textiles and refining of ores.
In the food industry for the preparation of glucose from starch.
In Medicine: As a component of gastric juice, it helps in digestion.
Key Differences to Remember
HCl (Gas): Dry, has no acidic property (does not turn blue litmus red).
Hydrochloric Acid (Solution): Aqueous solution, shows all acidic properties.
This chapter is crucial for understanding the preparation and behaviour of a key industrial acid and its gaseous form.
EXERCISE
1) Draw a labelled diagram for the laboratory preparation of hydrogen chloride gas. Give the balanced equation for the reaction.
Ans: Laboratory Preparation of Hydrogen Chloride Gas
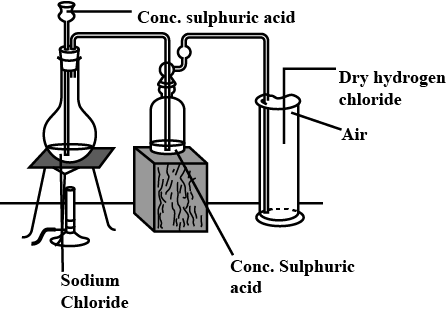
(A) Labelled Diagram:
A simple diagram would show:
Round-bottom flask: Contains the reactants (sodium chloride and concentrated sulphuric acid).
Thistle funnel: Dipping into the reaction mixture to add concentrated sulphuric acid.
Gas delivery tube: For leading the evolved HCl gas.
Gas jar or beaker: Placed upside-down in a water trough. The gas jar is used for collecting the gas by upward displacement of air because HCl gas is denser than air.
Water trough: Holds the gas jar.
(B) Balanced Equation:
The reaction is between sodium chloride and concentrated sulphuric acid.
NaCl (s) + H2SO4(l)→NaHSO4(s) + HCl (g)NaCl (s) + H 2SO4 (l)→NaHSO 4(s) + HCl(g)
2) Name the drying agents:
(a) used in drying hydrogen chloride gas.
(b) phosphorus pentoxide and calcium oxide are good drying agent but they cannot be used to dry hydrogen chloride gas. Why?
Ans: (a) Drying agents for hydrogen chloride gas:
Concentrated sulfuric acid or anhydrous calcium chloride can be used to dry hydrogen chloride gas.
(b) Reason phosphorus pentoxide and calcium oxide cannot be used:
Phosphorus pentoxide is acidic and reacts chemically with hydrogen chloride gas.
Calcium oxide is basic and also reacts with the acidic hydrogen chloride gas, forming calcium chloride and water.
Thus, they cannot be used as drying agents for hydrogen chloride.
3) Explain why:
(a) Anhydrous HCl is a poor conductor while aqueous HCl is an excellent conductor.
(b) When the stopper of a bottle full of hydrogen chloride gas is opened there are fumes in the air.
(c) A solution of hydrogen chloride in water turns blue litmus red, and conducts electricity, while a solution of the same gas in toluene: (i) has no effect on litmus, and (ii) does not conduct electricity.
(d) thick white fumes are formed when a glass rod dipped in NH2OH is brought near the mouth of a bottle full of HCl gas.
(e) dry hydrogen chloride gas does not affect a dry strip of blue litmus paper but it turns red in the presence of a drop of water.
(f) hydrogen chloride gas is not collected over water.
Ans: (a) Anhydrous HCl is a poor conductor while aqueous HCl is an excellent conductor.
Anhydrous HCl consists of covalent molecules with no free ions to conduct electricity.In water, HCl ionizes completely to form H⁺ (or H₃O⁺) and Cl⁻ ions. These mobile ions are responsible for excellent electrical conduction.
(b)Hydrogen chloride gas is highly soluble in water and has a strong affinity for moisture.When the stopper is opened, HCl gas diffuses out and reacts with water vapour present in the air, forming tiny droplets of hydrochloric acid. These suspended droplets are visible as white fumes.
(c) A solution of hydrogen chloride in water turns blue litmus red and conducts electricity, while in toluene:
(i) has no effect on litmus: In toluene, HCl remains as covalent molecules and does not ionize to release H⁺ ions, which are necessary to turn blue litmus red.
(ii) does not conduct electricity: Since no ions are formed in toluene, there are no charge carriers to conduct electricity.
(d) Thick white fumes are formed when a glass rod dipped in NH₄OH is brought near the mouth of a bottle full of HCl gas.
Ammonia gas (NH₃) evaporates from ammonium hydroxide (NH₄OH).This ammonia gas reacts with the hydrogen chloride (HCl) gas to form fine white solid particles of ammonium chloride (NH₄Cl). These suspended particles appear as thick white fumes.
(e) Dry hydrogen chloride gas does not affect a dry strip of blue litmus paper but it turns red in the presence of a drop of water.Dry HCl gas does not contain H⁺ ions; it exists as molecules.The drop of water allows HCl to dissolve and ionize, producing H⁺ ions. These H⁺ ions are acidic and turn the blue litmus paper red.
(f)Hydrogen chloride gas is extremely soluble in water.If collected over water, it would dissolve rapidly in the water instead of accumulating in the gas jar, making collection impossible.
4) Write the main difference between hydrogen chloride gas and hydrochloric acid.
Ans: The main difference is that hydrogen chloride (HCl) is a gas, while hydrochloric acid is a solution made from that gas.
Hydrogen Chloride: This is the pure compound, existing as separate HCl molecules.
Hydrochloric Acid: This is created when hydrogen chloride gas dissolves in water. In water, the HCl molecules split into H⁺ ions (which make it a strong acid) and Cl⁻ ions.
In short: Hydrogen chloride is the gas; hydrochloric acid is the gas dissolved in water.
5) The given set up in the figure is for the preparation of an acid.
(a) Name the acid prepared by this method.
(b) name the reactants used.
(c) why an empty flask is used
(d) what is the drying agent used? Why is this drying agent chosen?
(e) what is the role of inverted funnel in the arrangement
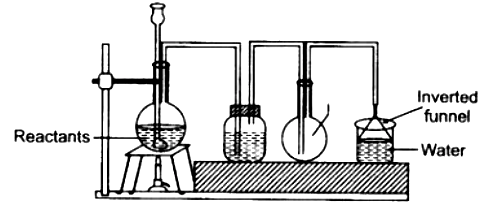
Ans: (a) Acid Prepared:
Hydrochloric acid (HCl).
(b) Reactants Used:
Sodium chloride (Common salt) and Concentrated sulphuric acid.
(c) Reason for Empty Flask:
The empty flask acts as a safety device. It prevents water (used for absorption) from being sucked back into the hot apparatus (jar containing reactants) in case the water flow is stopped or pressure changes.
(d) Drying Agent and Reason:
Drying Agent: Concentrated sulphuric acid.
Reason: It is chosen because it is a good dehydrating agent and it does not react with the hydrochloric acid gas.
(e) Role of Inverted Funnel:
The inverted funnel with its rim just dipping into water increases the surface area for the absorption of the HCl gas into the water. It also helps prevent back-suction of water into the delivery tube.
6) Write an equation for the reactions of aqueous hydrochloric acid on:
(a) silver nitrate solution
(b) magnesium foil
(c) caustic soda solution
(d) zinc carbonate
(e) lead nitrate solution
(f) copper oxide
Ans: (a) With silver nitrate solution
A white precipitate of silver chloride is formed.
AgNO₃(aq) + HCl(aq) → AgCl(s) + HNO₃(aq)
(b) With magnesium foil
Hydrogen gas is evolved with a pop sound.
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
(c) With caustic soda solution
A neutralization reaction occurs, producing salt and water.
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
(d) With zinc carbonate
Effervescence occurs due to the release of carbon dioxide gas.
ZnCO₃(s) + 2HCl(aq) → ZnCl₂(aq) + H₂O(l) + CO₂(g)
(e) With lead nitrate solution
A white precipitate of lead chloride is formed.
Pb(NO₃)₂(aq) + 2HCl(aq) → PbCl₂(s) + 2HNO₃(aq)
(f) With copper oxide
The black copper oxide dissolves, forming a blue-green solution.
CuO(s) + 2HCl(aq) → CuCl₂(aq) + H₂O(l)
7) (a) Name an element which reacts with hydrogen to form a compound which is strongly acidic in water.
(b) Explain why dilute hydrochloric acid cannot be concentrated by boiling beyond 22.2%.
Ans: (a) Chlorine
Chlorine reacts with hydrogen to form hydrogen chloride (HCl), which dissolves in water to form hydrochloric acid—a strong acid.
(b)Hydrochloric acid forms a constant boiling mixture (azeotrope) with water at approximately 22.2% HCl by mass.
Upon boiling, this mixture evaporates without changing composition, so further concentration beyond 22.2% is not possible by simple boiling.
8) Hydrochloric acid contains (i) hydrogen (ii) chlorine. Prove it. Write equations for the reactions.
Ans: (i) Proof it Contains Hydrogen
When active metals like zinc react with dilute hydrochloric acid, hydrogen gas is evolved. This gas can be identified by its characteristic ‘pop’ sound when a burning splint is brought near it.
Reaction:
Zn(s)+2HCl(aq)→ZnCl2(aq)+H2(g)↑Zn(s)+2HCl(aq) ZnClX 2(aq)+HX2(g)↑
The ‘pop’ test confirms the gas is hydrogen, proving HCl contains hydrogen.
(ii) Proof it Contains Chlorine
Hydrochloric acid contains chloride ions (Cl⁻). When silver nitrate solution is added, a thick, white precipitate of silver chloride is formed, which is insoluble in nitric acid. This is a standard test for chloride ions.
Reaction:
HCl(aq)+AgNO3(aq)→AgCl(s)↓+HNO3(aq)HCl(aq)+AgNOX (aq) AgCl(s)↓+HNOX (aq)
The formation of the white precipitate (AgCl) proves the original acid contained chlorine.
9) Name:
(a) black metallic oxide which reacts with hydrochloric acid to give a coloured solution.
(b) two colourless gases, which when mixed produce a white solid.
(c) two gases which chemically combine to form a liquid
(d) a chloride which is soluble in excess of ammonium hydroxide
(e) the chemical in which gold can be dissolved
(f) the experiment which demonstrates that hydrogen chloride is soluble in water.
(g) the gas produced when chlorine water is exposed to sunlight
Ans: (a) Manganese dioxide (MnO₂)
It reacts with HCl to form a green MnCl₂ solution.
(b) Ammonia (NH₃) and Hydrogen chloride (HCl)
They combine to form white ammonium chloride (NH₄Cl) solid.
(c) Hydrogen (H₂) and Oxygen (O₂)
They combine to form water (H₂O).
(d) Copper(II) chloride (CuCl₂)
Forms a soluble complex [Cu(NH₃)₄]²⁺ in excess NH₄OH.
(e) Aqua regia
A mixture of concentrated nitric acid and hydrochloric acid (in 1:3 ratio).
(f) Fountain experiment
Shows high solubility of HCl in water.
(g) Oxygen (O₂)
Formed when chlorine water decomposes in sunlight.
10) Give reasons for the following:
(a) An aqueous solution of chlorine in acidic in nature Hint : Cl2 + H2O ⟶ HCl +HClO
(b) silver nitrate solution can be used to distinguish HCl from HNO3
Ans: (a) When chlorine dissolves in water, it undergoes a disproportionation reaction:
Cl₂ + H₂O → HCl + HClO
This reaction produces hydrochloric acid (HCl), which is a strong acid, and hypochlorous acid (HClO), a weak acid. The presence of these acids, particularly the strong HCl, releases H⁺ ions into the solution, making it acidic.
(b) Silver nitrate solution can be used to distinguish HCl from HNO₃
Silver nitrate (AgNO₃) reacts with hydrochloric acid (HCl) to form a curdy white precipitate of silver chloride (AgCl), which is insoluble in water.
AgNO₃ + HCl → AgCl (↓) + HNO₃
Nitric acid (HNO₃), being a strong acid, does not form any precipitate with silver nitrate. It simply results in a mixture of soluble salts and no visible change occurs. Thus, the formation of a white precipitate distinguishes HCl from HNO₃.
11) Solution A reacts with an acid B (which gives greenish yellow gas on reacting with oxidizing agents like Pb3O4 to give white precipitate C insoluble in nitric acid but soluble in ammonium hydroxide. Name A, B and C.
Ans: A is Silver nitrate
B is Hydrochloric acid
C is Silver chloride
12) Complete the following reactions and balance them.
(a) NH4OH + HCl ⟶
(b) NaHSO3 + HCl ⟶
(c) Pb(NO3)2 +HCl ⟶
(d) Pb3O4 + HCl ⟶
(e) Zn+ 2HCl ⟶
(f) Ca(HCO3)2 + 2HCl ⟶
Ans:
(a) NH4OH + HCl ⟶ NH4Cl + H2O
(b) NaHSO3 + HCl ⟶ NaCl + H2O + SO2
(c) Pb(NO3)2 +2HCl ⟶ PbCl2 +2HNO3
(d) Pb3O4 + 8HCl ⟶ 3PbCl2 +4H2O +Cl2
(e) Zn+ 2HCl ⟶ ZnCl2 + H2
(f) Ca(HCO3)2 + 2HCl ⟶ CaCl2 + 2H2O + 2CO2
13) How will the action of dilute hydrochloric acid enable you to distinguish between the following:
(a) Sodium carbonate and sodium sulphite
(b) sodium thiosulphate and sodium sulphite
Ans: (a) Distinguishing between Sodium Carbonate and Sodium Sulphite
Add dilute hydrochloric acid to both solids or solutions.
Sodium Carbonate (Na₂CO₃): It will produce brisk effervescence due to the release of carbon dioxide (CO₂) gas, which is odorless.
Sodium Sulphite (Na₂SO₃): It will also produce effervescence, but it releases sulphur dioxide (SO₂) gas, which has a sharp, choking smell like burning sulphur.
Identification: The gas with the pungent smell identifies sodium sulphite, while the odorless gas identifies sodium carbonate.
(b) Distinguishing between Sodium Thiosulphate and Sodium Sulphite
Add dilute hydrochloric acid to both solids or solutions.
Sodium Thiosulphate (Na₂S₂O₃): It reacts to produce a milky yellow precipitate of sulphur along with the smell of sulphur dioxide. The solution turns cloudy.
Sodium Sulphite (Na₂SO₃): It reacts to produce only sulphur dioxide gas with a pungent smell, but no precipitate is formed.
Identification: The formation of a yellow precipitate identifies sodium thiosulphate, while the absence of a precipitate identifies sodium sulphite.
14) Give three distinct test [apart from using an indicator] you would carry out with a solution of HCl to illustrate the typical properties of an acid.
Ans: Here are three distinct tests for HCl to show typical acid properties:
Reaction with a metal
Add a small piece of zinc or magnesium to the HCl solution. You will observe brisk effervescence due to the liberation of hydrogen gas.
Reaction with a metal carbonate
Add a small amount of sodium carbonate or calcium carbonate to the HCl solution. You will see brisk effervescence due to the release of carbon dioxide gas.
Reaction with a base
Add a small amount of copper(II) oxide to the warm HCl solution. The black solid will dissolve, forming a clear blue solution of copper(II) chloride.
15) MnO2, PbO2 and red lead react with conc. HCl acid liberates Cl2. What is the common property being shown by these metal oxides?
Ans: The common property shown by MnO₂, PbO₂, and red lead (Pb₃O₄) is that they are all strong oxidizing agents.When reacted with concentrated HCl, these metal oxides provide oxygen or accept electrons, which oxidizes the chloride ions (Cl⁻) from the acid to chlorine gas (Cl₂).
16) Giving reasons state which of the two- a solution of HCl in water or in toluene is an electrolyte.
Ans: A solution of HCl in water is an electrolyte, while a solution in toluene is not.
Reasons:
- In Water: Water is a polar solvent. It ionizes HCl molecules into free-moving H⁺ (or H₃O⁺) and Cl⁻ ions. These mobile ions allow the solution to conduct electricity, making it an electrolyte.
- In Toluene: Toluene is a non-polar solvent. It cannot ionize HCl. The HCl remains as neutral, covalently bonded molecules. Since there are no free ions to carry electric current, the solution is a non-electrolyte.
17) Convert two soluble metallic nitrates to insoluble metallic chlorides using dil. HCl.
Ans: To convert two soluble metallic nitrates to insoluble metallic chlorides using dilute HCl, you can use the following reactions:
For Silver Nitrate:
When dilute Hydrochloric acid (HCl) is added to Silver nitrate solution (AgNO₃), a double displacement reaction occurs.A white precipitate of Silver chloride (AgCl), which is insoluble in water, is formed.
Reaction: AgNO₃(aq) + HCl(aq) → AgCl(s)↓ + HNO₃(aq)
For Lead Nitrate:
When dilute Hydrochloric acid (HCl) is added to Lead nitrate solution (Pb(NO₃)₂), a similar reaction takes place.A white precipitate of Lead chloride (PbCl₂), which is sparingly soluble (insoluble in cold water), is formed.
Reaction: Pb(NO₃)₂(aq) + 2HCl(aq) → PbCl₂(s)↓ + 2HNO₃(aq)
In both cases, the insoluble chloride salt can be separated from the mixture by filtration.
18) State the composition of aqua regia. State which component is the oxidizing agent in aqua regia.
Ans: Composition of Aqua Regia:
Aqua regia is a mixture of concentrated hydrochloric acid (HCl) and concentrated nitric acid (HNO₃) in a 3:1 volume ratio.
Oxidizing Agent:
The oxidizing agent in aqua regia is nitric acid (HNO₃). It oxidizes chlorine ions from HCl to produce chlorine and nascent chlorine, which are responsible for dissolving noble metals like gold and platinum.
19) Convert hydrochloric acid to nascent chlorine.
Ans: To convert hydrochloric acid (HCl) to nascent chlorine, simply react it with a strong oxidizing agent.A common and simple method is to react hydrochloric acid with potassium permanganate (KMnO₄).
The reaction is:
2KMnO₄ + 16HCl → 2KCl + 2MnCl₂ + 8H₂O + 5Cl₂
The chlorine gas (Cl₂) produced is nascent chlorine, which is highly reactive.
20) Study the flow chart and give balanced equations with conditions for the conversions A, B, C and D.
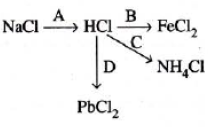
Ans: Here are the balanced equations for the conversions A, B, C, and D based on the standard flowchart for the Contact Process.
A: S + O₂ → SO₂
Conversion: Sulphur to Sulphur dioxide
Conditions: Burning in air.
B: 4FeS₂ + 11O₂ → 2Fe₂O₃ + 8SO₂
Conversion: Iron pyrites to Sulphur dioxide
Conditions: Roasting in air.
C: 2SO₂ + O₂ ⇌ 2SO₃
Conversion: Sulphur dioxide to Sulphur trioxide
Conditions: Temperature ~720K, Pressure ~2 atm, Vanadium pentoxide (V₂O₅) catalyst.
D: SO₃ + H₂SO₄ → H₂S₂O₇ (Oleum)
Conversion: Sulphur trioxide to Oleum
Conditions: Absorption in concentrated sulphuric acid. (Oleum is then diluted to get H₂SO₄).
Question (2004) 21: A solution of hydrogen chloride in water is prepared. The following substances are added to separate portions of the solution:
Adding different substances to a hydrochloric acid (HCl) solution will produce different observable reactions because HCl is a strong acid and a source of chloride ions (Cl⁻).
Ans: Zinc (a metal): Effervescence (bubbles) of hydrogen gas (H₂) will be observed.
Zn + 2HCl → ZnCl₂ + H₂
Sodium Carbonate: Vigorous effervescence of carbon dioxide gas (CO₂) will be observed.
Na₂CO₃ + 2HCl → 2NaCl + H₂O + CO₂
Silver Nitrate: A thick, white precipitate of silver chloride (AgCl) will form.
AgNO₃ + HCl → AgCl (white ppt) + HNO₃
These tests confirm the acidic nature and the presence of chloride ions in the solution.