1. Introduction to Metals & Ores
Metals are found in the Earth’s crust either as free elements (like gold) or more commonly as compounds called minerals. A mineral from which a metal can be extracted profitably and conveniently is called an ore. The impurities present in the ore are known as gangue.
2. Extraction of Metals
The process of extracting metals from their ores involves three major steps:
Concentration of Ore (Enrichment): Removing the gangue to increase the metal content. Common methods include:
Hydraulic Washing (Gravity Separation): Using water to separate heavier ore from lighter gangue.
Froth Floatation: Used for sulphide ores where the ore particles are separated by creating a froth.
Conversion to Metal Oxide: The concentrated ore is often converted to its oxide because it is easier to reduce oxides to metals. This is done by:
Calcination: Heating the ore strongly in the absence of air (for carbonate or hydrated ores).
Roasting: Heating the ore strongly in the presence of air (for sulphide ores).
Reduction to Free Metal: The metal oxide is reduced to the metallic state. The common method is smelting, where the oxide is heated with a reducing agent like coke (carbon). For highly reactive metals like Aluminium, a different method called electrolytic reduction is used.
3. The Reactivity Series & Extraction Method
The position of a metal in the Reactivity Series determines how it is extracted:
Metals at the top (K, Na, Ca, Mg, Al): Are very reactive and are extracted by electrolysis of their molten chlorides or oxides.
Metals in the middle (Zn, Fe, Pb, etc.): Are moderately reactive and are extracted by reduction with carbon (coke).
Metals at the bottom (Cu, Hg, Ag): Are less reactive. Some can be extracted just by heating (e.g., Mercury from Cinnabar) or by auto-reduction.
4. Refining of Metals
The metal obtained after reduction is often impure. It is purified, most commonly by electrolytic refining. In this process, the impure metal is made the anode, a pure strip of the same metal is the cathode, and a salt solution of the metal is the electrolyte. Pure metal deposits on the cathode.
5. Corrosion
The slow process of decay or destruction of metals due to air and moisture is called corrosion (e.g., rusting of iron). It is an electrochemical process. Prevention methods include galvanization (coating with zinc), tin-plating, painting, greasing, and making alloys.
EXERCISE- 7 (A)
1)Name the three classes in which elements are classified. Which was the first metal used by man?
Ans: Three classes in which elements are classified are: Metals ,
Non-metals and Metalloids
Copper was the first metal used by man.
2) Name the metal which is a constituent of:
(a) Blood pigment, (b) plant pigment
Ans: (a) Iron
(b) Magnesium
3) Give the importance of the following in living beings:
(a) Nitrogen, (b) Hydrogen, (c) carbon
Ans: (a) Nitrogen
Protein Builder: It is a fundamental component of amino acids, the building blocks of proteins. Proteins are essential for muscle, tissue, enzymes, and antibodies.
Genetic Code: Nitrogen is a key element in nucleic acids like DNA and RNA, which carry the genetic instructions for all life processes.
Chlorophyll: In plants, it is a vital part of the chlorophyll molecule, which is necessary for photosynthesis.
(b) Hydrogen
Water and Solvent: Hydrogen, combined with oxygen, forms water (H₂O), which is the universal solvent and medium for all biochemical reactions in living organisms.
Energy Carrier: It plays a central role in energy transfer, most notably in the electron transport chain during cellular respiration, where it helps produce ATP (the energy currency of the cell).
Acid-Base Balance: Hydrogen ions (H⁺) determine the pH of bodily fluids, which is critical for the proper functioning of enzymes and metabolic processes.
(c) Carbon
The Backbone of Life: Carbon atoms have a unique ability to form four strong bonds, allowing them to create long chains and complex rings. This makes carbon the fundamental skeleton for all organic molecules.
Essential Molecules: It is the core element of all major biomolecules, including carbohydrates, lipids, proteins, and nucleic acids. Life as we know it is carbon-based.
4) Name the metal and non-metal present in abundance in the earth crust.
Ans: The most abundant metal in the Earth’s crust is aluminium.
The most abundant non-metal in the Earth’s crust is oxygen.
5) Define metal and non-metal on the basis of electron loss or gain.
Ans: On the basis of electron loss or gain:
Metal: Metals are elements that tend to lose electrons easily from their outermost shell to form positive ions (cations). This is because they have typically 1, 2, or 3 valence electrons.
Non-metal: Non-metals are elements that tend to gain electrons easily into their outermost shell to form negative ions (anions). This is because they have typically 5, 6, or 7 valence electrons.
6) State the position of the following in the periodic table:
(a) Alkali metals,
(b) Alkaline earth metals
(c) Iron and zinc
(d) Aluminium
Ans: (a) Alkali Metals
The Alkali Metals are located in Group 1 of the periodic table. This is the first vertical column on the far left-hand side.
Key Members: This group includes elements like Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Caesium (Cs), and Francium (Fr).
Important Note: Hydrogen (H) is also placed in Group 1 because it has one valence electron, but it is not an alkali metal. It is a unique non-metal.
(b) Alkaline Earth Metals
The Alkaline Earth Metals are located just to the right of the alkali metals, in Group 2 of the periodic table.
Key Members: This family includes Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), and Radium (Ra).
(c) Iron and Zinc
Iron and Zinc are both transition metals. They are located in the central block of the periodic table, between Group 2 and Group 13.
Iron (Fe): You can find Iron in Period 4, Group 8.
Zinc (Zn): Zinc is located right next to the transition metals in Period 4, Group 12. While it is in the d-block, Zinc has a full d-orbital and is sometimes considered a post-transition metal.
(d) Aluminium
Aluminium is found in Group 13 and Period 3 of the periodic table.
Classification: It is the most well-known member of the Boron Group. It is classified as a post-transition metal due to its metallic properties, even though it is placed in the p-block of the table.
7) Give the general characteristics of:
(a) Alkali metals,
(b) Alkaline earth metals with reference to
(i) bonding
(ii) action of air
(iii) action of water
(iv) action of acid
Ans: (a) Alkali Metals (Group 1: Li, Na, K, Rb, Cs, Fr)
(i) Bonding:
They have one electron in their outermost shell. They readily lose this electron to form ionic (electrovalent) bonds, achieving a stable noble gas configuration. This results in the formation of M⁺ ions.
(ii) Action of Air:
They are very reactive with air. Freshly cut surfaces are shiny but quickly tarnish due to reaction with oxygen and moisture, forming a layer of oxide, hydroxide, and finally carbonate. They are stored in inert liquids like kerosene to prevent this.
(iii) Action of Water:
They react violently with cold water to produce hydrogen gas and the corresponding metal hydroxide. The reaction is highly exothermic and can ignite the hydrogen (e.g., sodium melts and dances on water; potassium burns with a lilac flame).
(iv) Action of Acid:
They react explosively with acids to produce salt and hydrogen gas. The reaction is much more vigorous than with water due to the higher concentration of H⁺ ions.
(b) Alkaline Earth Metals (Group 2: Be, Mg, Ca, Sr, Ba, Ra)
(i) Bonding:
They have two electrons in their outermost shell. They lose these two electrons to form ionic bonds, resulting in M²⁺ ions. Their compounds are generally less ionic than those of alkali metals due to higher charge density.
(ii) Action of Air:
They are reactive but less so than alkali metals. They slowly tarnish in air, forming a protective oxide layer on their surface (e.g., magnesium’s dull appearance). On heating, they burn vigorously to form oxides and nitrides.
(iii) Action of Water:
Their reactivity with water increases down the group.Beryllium does not react with water.Magnesium reacts very slowly with cold water but readily with steam to form magnesium oxide and hydrogen.Calcium and the metals below it react with cold water, though less violently than alkali metals, to form hydroxides and hydrogen.
(iv) Action of Acid:
They react readily with acids to produce the corresponding salt and hydrogen gas. The reaction is generally fast and exothermic but typically less violent than that of alkali metals.
8) What are metalloids: Give examples.
Ans: Metalloids are a unique category of elements that share a blend of metallic and non-metallic traits.Their most important property is being semiconductors. This means they can control electrical flow, making them vital for computers and electronics. In appearance, they can be shiny like metals or dull like non-metals.
Well-known metalloids are Boron, Silicon, Germanium, Arsenic, Antimony, and Tellurium.
9) Why is hydrogen placed with alkali metals?
Ans: Hydrogen is placed with alkali metals in Group 1 for the following key reasons:
Similar Electronic Configuration: Like alkali metals (Li, Na, K, etc.), hydrogen has a single electron in its outermost shell. Alkali metals have the configuration ns¹, and hydrogen has 1s¹.
Similar Behaviour:
Electropositive Nature: Both hydrogen and alkali metals tend to lose their single valence electron to form positive ions (H⁺ and M⁺, e.g., Na⁺).
Combination with Anions: They form similar compounds, such as halides (HCl, NaCl) and oxides (H₂O, Na₂O).
However, hydrogen also shows properties very different from alkali metals, such as forming negative ions (H⁻) and diatomic gas molecules (H₂), which is why its position in the periodic table is sometimes considered anomalous.
10) Name: (a) a liquid non-metal,
(b) a metal with dull appearance
(c) a metal with low melting and boiling points
(d) a non-metal with high m.p & b.p
(e) a metal which can float on water
(f) a metal which can be cut with a knife.
(g) a metal which is a bad conductor of heat and electricity
(h) a non-metal which is ductile
(i) a non- metal used in alloys
(j) a non-malleable metal
Ans: (a) Bromine
(b) Lead
(c) Gallium
(d) Carbon
(e) Sodium
(f) Sodium
(g) Tungsten
(h) Carbon fibre
(i) Carbon
(j) Mercury
11) Distinguish between metals and non metals on the basis of:
(i) ion formation,
(ii) discharge of ions,
(iii) nature of oxide formed,
(iv) oxidizing and reducing property,
(v) reaction with acids.
Ans: (i) Ion Formation
Metals: Tend to lose electrons from their outermost shell to form positive ions (cations).
Example: Sodium (Na) loses 1 electron to form Na⁺.
Non-metals: Tend to gain electrons into their outermost shell to form negative ions (anions).
Example: Chlorine (Cl) gains 1 electron to form Cl⁻.
(ii) Discharge of Ions
Metals: During electrolysis, their positive ions (cations) are discharged at the cathode (negative electrode).
Non-metals: During electrolysis, their negative ions (anions) are discharged at the anode (positive electrode).
(iii) Nature of Oxide Formed
Metals: Generally form basic oxides.
Example: Sodium oxide (Na₂O) turns red litmus paper blue.
Non-metals: Generally form acidic oxides.
Example: Sulphur dioxide (SO₂) turns blue litmus paper red.
(iv) Oxidizing and Reducing Property
Metals: Act as reducing agents because they lose electrons easily (they get oxidized).
Non-metals: Act as oxidizing agents because they gain electrons easily (they get reduced).
(v) Reaction with Acids
Metals: Generally react with dilute acids (like HCl, H₂SO₄) to produce hydrogen gas.
Example: Zn + 2HCl → ZnCl₂ + H₂
Non-metals: Do not react with dilute acids to produce hydrogen gas.
12) (a) Na _____ —>Na+
(b) N+ _____ —>N3-
(c) Cl +e- —> _____
(d) Mg -_____ —> Mg2+
(e) M+ HCl —>MCl2 +_____
(f) Mg +H2SO4 —> _____ + _____
Ans: (a) Na e- —>Na+
(b) N+ 3e- —>N3-
(c) Cl +e- —> Cl-
(d) Mg -2e- —> Mg2+
(e) M+ HCl —>MCl2 + H2
(f) Mg +H2SO4 —> MgSO4 + H2
13) Select from the following is: Fe2O3, , NO, PbO, Mn2O7
(a) Basic oxide………..
(b) Amphoteric oxide …………
(c) Acidic oxide ………………
(d) Neutral oxide ……………..
Ans: (a) Fe2O3
(b) PbO
(c) Mn2O7
(d) NO
14) Take an element from an alkali metal and one from an alkaline earth metal and write an equation for their action with:
(a) Hydrochloric acid,
(b) Oxygen
(c) Sulphuric acid
(d) Water.
Ans: (a) Action with Hydrochloric Acid
Sodium (Na): Reacts violently, producing hydrogen gas and salt.
Equation: 2Na(s) + 2HCl(aq) → 2NaCl(aq) + H₂(g)
Calcium (Ca): Reacts vigorously, producing hydrogen gas and salt.
Equation: Ca(s) + 2HCl(aq) → CaCl₂(aq) + H₂(g)
(b) Action with Oxygen
Sodium (Na): Burns vigorously to form a white solid (oxide/peroxide).
Equation: 4Na(s) + O₂(g) → 2Na₂O(s) (Mainly sodium oxide)
Calcium (Ca): Burns with a brilliant flame to form a white solid (oxide).
Equation: 2Ca(s) + O₂(g) → 2CaO(s)
(c) Action with Sulphuric Acid
Sodium (Na): Reacts violently with dilute acid. With concentrated acid, the reaction is very dangerous.
Equation (with dilute acid): 2Na(s) + H₂SO₄(aq) → Na₂SO₄(aq) + H₂(g)
Calcium (Ca): Reacts vigorously with dilute sulphuric acid. With concentrated acid, a protective layer of calcium sulphate stops the reaction.
Equation (with dilute acid): Ca(s) + H₂SO₄(aq) → CaSO₄(aq) + H₂(g)
(d) Action with Water
Sodium (Na): Reacts violently, melting and darting on the water surface, producing hydrogen gas and an alkali.
Equation: 2Na(s) + 2H₂O(l) → 2NaOH(aq) + H₂(g)
Calcium (Ca): Reacts less violently than sodium, producing hydrogen gas and a sparingly soluble alkali.
Equation: Ca(s) + 2H₂O(l) → Ca(OH)₂(aq) + H₂(g)
EXERCISE 7 (B)
1) Name: (a) Two metals which are liquid at room temperature
(b) two metals which are soft
(c) a metal which lacks ductility
(d) a non metal which is lustrous
(e) a non metal which conducts electricity
(f) a metal which is brittle
(g) two non metals which are monoatomic
(h) two metallic oxides which are acidic
(i) two metallic oxides which are amphoteric
(j) two metals which react with cold water,
(k) the compound responsible for green deposit on the surface of copper
(l) the most abundant metal and the most abundant non=metal
(m) a non metal which can form a positive ion
(n) a non-metal which shows reducing property
(o) a metal whose oxide is reduced only by carbon
Ans: (a) Mercury and gallium
(b) Sodium and potassium
(c) Mercury
(d) Iodine
(e) Graphite
(f) Zinc
(g) Neon , Argon
(h) CrO3 , Mn2O7
(i) Al2O3,PbO
(j) Potassium , sodium
(k) Basic copper(II) sulphate
(l) Aluminium , Oxygen
(m) Hydrogen
(n) Carbon
(o) Iron
2) Explain how the activity series accounts for each of the following:
(a) occurrence of metals
(b) tendency to corrosion
(c) reaction with water
(d) reaction with acids
Ans: (a) Occurrence of Metals
The activity series explains that highly reactive metals (like potassium, sodium, calcium) have a strong tendency to form compounds. Therefore, they are rarely found in nature as pure, native metals. In contrast, less reactive metals (like gold, silver, platinum) have little tendency to combine and are often found in their free, metallic state.
(b) Tendency to Corrosion
Corrosion is essentially the reaction of a metal with its environment (e.g., oxygen, water). The activity series shows that metals higher on the list are more readily oxidized, meaning they corrode much faster. A metal like iron corrodes easily, while a metal like gold does not corrode at all under normal conditions.
(c) Reaction with Water
The activity series determines how vigorously a metal reacts with water. Only metals above hydrogen in the series react. Highly reactive metals (e.g., potassium, sodium) react violently with cold water. Less reactive metals (e.g., magnesium) react slowly with steam. Metals below hydrogen (e.g., copper, silver) do not react with water.
(d) Reaction with Acids
A metal will react with a common acid (like hydrochloric acid) only if it is positioned above hydrogen in the activity series. This is because these metals can displace hydrogen ions from the acid. Metals below hydrogen, such as copper and silver, have no reaction with these acids as they cannot displace the hydrogen.
3) Give the balanced reactions for the following:
(a) Sodium is dropped in water
(b) Magnesium reacts with boiling water
(c) Red hot iron reacts with steam
(d) Iron reacts with dilute HCI
Ans: (a) Sodium is dropped in water
2Na(s)+2H2O(l)→2NaOH(aq)+H2(g)2Na(s)+2H 2O(l)→2NaOH(aq)+H2(g)
(b) Magnesium reacts with boiling water
Mg(s)+2H2O(l)→Mg(OH)2(aq)+H2(g)Mg(s)+2H2O(l)→Mg(OH) 2(aq)+H2(g)
(c) Red hot iron reacts with steam
3Fe(s)+4H2O(g)→Fe3O4(s)+4H2(g)3Fe(s)+4H 2O(g)→Fe 3O4 (s)+4H2 (g)
(d) Iron reacts with dilute HCl
Fe(s)+2HCl(aq)→FeCl2(aq)+H2(g)Fe(s)+2HCl(aq)→FeCl 2(aq)+H2(g)
4) Give a short account of the heating effect on metal carbonates based on the activity series.
Ans: The effect of heat on a metal carbonate depends directly on the metal’s position in the reactivity series.
1. Carbonates of Less Reactive Metals (e.g., Copper, Silver, Gold, Lead)
These carbonates are thermally unstable and decompose easily when heated. They break down into the metal oxide and carbon dioxide gas.
For example: Copper carbonate (green) decomposes to black copper oxide and carbon dioxide.
2. Carbonates of Highly Reactive Metals (e.g., Sodium, Potassium, Calcium)
These carbonates are highly stable and require an immense amount of heat to decompose—so much that it is not practically feasible with a simple Bunsen burner. They generally do not decompose on heating.
3. Carbonates of Middle-Order Metals (e.g., Zinc, Iron, Magnesium)
These carbonates decompose, but only when heated very strongly. For instance, zinc carbonate decomposes to zinc oxide and carbon dioxide upon strong heating.
In summary: The general rule is that the higher a metal is in the reactivity series, the more stable its carbonate is to heat. The lower the metal is, the more readily its carbonate decomposes upon heating.
5) (a) Why are alkali metals kept in kerosene oil?
(b) What is:
(i) basic lead carbonate and
(ii) brown powder deposit on iron?
(c) Why is hydrogen kept in the metal activity series?
Ans:
(a)Alkali metals are stored in kerosene oil to prevent contact with air and moisture, avoiding violent reactions and fire hazards.
(b)(i) Basic lead carbonate – 2PbCO₃·Pb(OH)₂ (white lead).
(ii) The brown powder on iron is rust – hydrated ferric oxide (Fe₂O₃·xH₂O).
(c)Hydrogen is included in the metal activity series because it can lose an electron to form H⁺ ions, helping compare how metals displace hydrogen from acids.
6) Give the effect of heat on metal oxides based on the activity series.
Ans: Effect of Heat on Metal Oxides:
The behavior of a metal oxide when heated depends on its position in the reactivity series:
Oxides of Potassium, Sodium, Calcium, Aluminum (Top of the series):
These metal oxides are very stable and do not decompose on heating. It is extremely difficult to break their bonds and extract the metal.
Oxides of Zinc, Iron, Lead, Copper (Middle to lower series):
These metal oxides are unstable and decompose on strong heating. They break down to produce the metal and oxygen gas.
General Reaction: Metal Oxide → Metal + Oxygen
Oxides of Silver, Gold, Mercury (Bottom of the series):
These metal oxides are very unstable. They decompose even with mild heating (some, like silver and gold oxides, decompose at room temperature).
In short: The stability of a metal oxide decreases down the reactivity series. Oxides of highly reactive metals are stable to heat, while oxides of less reactive metals decompose easily.
7) Metal A has an electronic configuration of 2, 8, 1 and metal B has 2, 8, 8, 2 which is more reactive metal.
(a) Identify A and B and give their reactions with dil HCL and dil H2SO4
(b) Give the effect of heat on their: (i) oxides (ii) hydroxide (iii) carbonates (iv) nitrates
Ans: The behavior of a metal oxide when heated depends on its position in the reactivity series:
Oxides of Potassium, Sodium, Calcium, Aluminum (Top of the series):
These metal oxides are very stable and do not decompose on heating. It is extremely difficult to break their bonds and extract the metal.
Oxides of Zinc, Iron, Lead, Copper (Middle to lower series):
These metal oxides are unstable and decompose on strong heating. They break down to produce the metal and oxygen gas.
General Reaction: Metal Oxide → Metal + Oxygen
Oxides of Silver, Gold, Mercury (Bottom of the series):
These metal oxides are very unstable. They decompose even with mild heating (some, like silver and gold oxides, decompose at room temperature).
In short: The stability of a metal oxide decreases down the reactivity series. Oxides of highly reactive metals are stable to heat, while oxides of less reactive metals decompose easily.
8) (a) The table below compares some properties of metals and non-metals. Write down the missing statements (i) to (iv) :
| Metals | Non-metals |
| (i) ………………….. | Poor conductors of heat |
| (ii) Malleable | |
| (iii) Form positive ions | |
| (iv) ………………… | Form acidic oxides |
(b) How many valence electrons are present in:
(i) metals and (ii) non-metals?
Ans: (a)
| Metals | Non-metals |
| (i) Good conductors of heat | Poor conductors of heat |
| (ii) Malleable | Non-Malleable |
| (iii) Form positive ions | Forms negative ions |
| (iv) Form basic oxides | Form acidic oxides |
(b) (i) Valence Electrons in Metals:
Metals typically have a small number of electrons in their outermost shell. It is common for them to possess 1, 2, or 3 valence electrons. Because of this low count, metals tend to lose these outer electrons easily when they undergo chemical reactions.
(ii) Valence Electrons in Non-Metals:
They generally contain 5, 6, or 7 valence electrons. To achieve a stable electron arrangement, non-metals usually gain or share electrons during bonding.A helpful point to note is that hydrogen, with 1 valence electron, and helium, with 2, are common exceptions to this pattern.
9) What is corrosion? What are necessary conditions for corrosion?
Ans: Corrosion is a natural process where a refined metal is slowly damaged and broken down because of its reaction with the surrounding environment. A simple example we see every day is the rusting of iron, where a flaky, reddish-brown coating forms on the surface.
For corrosion—specifically the rusting of iron—to occur, two main things are needed:
Water or Moisture: The metal must be in contact with water or be in a damp environment.
Oxygen from the Air: The metal needs to be exposed to the oxygen present in the air.
Both water and oxygen must be present at the same time for rust to form. The process can also happen much faster if the water contains dissolved substances like salt or acids, as these make the water more conductive and speed up the chemical reaction.
10) State under what conditions corrosion is faster
Ans: Corrosion happens much faster when:
Water and Salt are Present: Moisture, especially salt water, greatly speeds up rusting.
The Environment is Acidic: Acids (like those in polluted rain) aggressively eat away at metals.
The Temperature is High: Heat accelerates the chemical reactions of corrosion.
The Metal is Stressed: Bent or strained areas of metal corrode more easily.
Certain Gases are in the Air: Gases like carbon dioxide or sulfur dioxide create acidic conditions that promote corrosion.
11) Corrosion can be an advantage in some case.Explain
Ans: Yes, corrosion can be beneficial. A key example is the green patina that forms on copper or bronze surfaces, such as on old statues and roofs.This patina acts as a stable, protective barrier. It seals the underlying metal from air and moisture, effectively preventing any deeper corrosion and preserving the metal for a long time.
12) What is rust? Give the equation for the formation of rust.
Ans: Rust is the reddish-brown flaky substance that forms on iron when it reacts with both oxygen and water from the air. This chemical reaction is a specific type of corrosion.
The process can be summarized as:
Iron + Oxygen + Water → Rust
The most important thing to remember is that both air (oxygen) and moisture (water) are essential for rusting to happen. If either one is absent, iron will not rust.
13) State two conditions necessary for rusting of iron.
Ans: For iron to rust, two things are absolutely necessary:
Moisture: There must be water or water vapour present. Even the moisture in the air is enough to start the process.
Air (Oxygen): The air around the iron must contain oxygen. Rusting is essentially a reaction between iron and oxygen, and water acts as a catalyst to make it happen.
If you can block the iron from coming into contact with either water or oxygen, you can successfully prevent it from rusting. Both conditions must be present together for rust to form.
14) How does the painting of an iron object prevent rusting?
Ans: Rusting is a chemical process that affects iron and steel, and it cannot happen without two key ingredients: the oxygen from the air and moisture (water). When both are present on the iron’s surface, they react with the metal to form rust, which is a flaky, weak compound.Painting works as a simple yet effective shield against this process. When a coat of paint is applied to an iron object, it dries to form a continuous, solid film over the entire surface.This protective paint layer acts as a physical barrier that performs two crucial functions:It seals the iron off from the moisture and water vapour in the surrounding air.It prevents oxygen in the atmosphere from making direct contact with the metal.By denying the iron access to both oxygen and water, the chemical reaction required for rusting is stopped. Therefore, as long as the painted coating remains intact without any chips, scratches, or cracks, the iron underneath will be safely preserved and will not rust.
15) What is galvanization? How does it protect iron from rusting?
Ans: Galvanization is a process of applying a protective zinc coating on iron or steel objects by dipping them into molten zinc.
It protects iron from rusting in two ways:
Barrier Protection: The zinc coating acts as a physical barrier, preventing air and moisture from reaching the iron surface.
Sacrificial Protection: Even if the coating is scratched, the zinc sacrifices itself (corrodes) instead of the iron. This happens because zinc is more reactive than iron.
16) A student has been collecting silver coins and copper coins. One day she observed a black coating on silver coins and a green coating on copper coins. Which chemical phenomenon is responsible for these coatings? Write the names of black and green coatings
Ans: The unwanted layers that form on metals, like the black on silver or the green on copper, are caused by a natural chemical process called corrosion. This happens when metals react chemically with elements in their surroundings, such as moisture or oxygen in the air, causing them to slowly break down.
To be more specific:
The black film that appears on items like silver coins and utensils is a compound called Silver Sulphide. This particular kind of corrosion is commonly known as tarnishing.The distinctive greenish-blue layer seen on copper items like old coins, statues, and roofs is a compound called Basic Copper Carbonate. This specific form of corrosion has the traditional name verdigris.
17) Aluminium is said to be more reactive than iron, towards oxygen (or air) yet iron undergoes corrosion to a greater extent than aluminum. Explain.
Ans: Aluminium is chemically more reactive than iron, meaning it has a stronger tendency to lose electrons and react with oxygen.However, when aluminium reacts with air, it forms a thin, strong, and tightly-adhered layer of aluminium oxide (Al₂O₃) on its surface. This layer is non-porous and impermeable, acting as a protective shield. It prevents further oxygen from reaching the metal underneath, effectively making the aluminium passive and stopping any further corrosion.In contrast, when iron corrodes, it forms hydrated ferric oxide (Fe₂O₃.xH₂O), which we know as rust. This rust layer is porous, flaky, and brittle. It peels off easily, exposing a fresh layer of iron to air and moisture, allowing the corrosion to continue until the entire piece of iron is weakened or destroyed.
18) Which metals do not corrode easily?
Ans: Metals that do not corrode easily are generally the noble metals and others that form a protective layer.
The most common examples are:
Gold (Au) and Platinum (Pt): These are known as ‘noble metals’ because they are largely unreactive. They do not react with air, water, or most chemicals, which is why they are used in high-quality jewellery and electronics without rusting or tarnishing.
Silver (Ag): While it is a noble metal, silver can slowly tarnish by reacting with sulphur compounds in the air, forming a black layer of silver sulphide. However, it does not corrode easily in the same way iron does.
Stainless Steel: This is not a pure metal but an alloy of iron, chromium, and nickel. The chromium in it reacts with oxygen to form a thin, invisible, and adherent layer of chromium oxide on the surface. This layer prevents further corrosion, making it highly resistant to rust.
Aluminium (Al): Aluminium is a very reactive metal, but it owes its corrosion resistance to a protective shield. It quickly forms a hard layer of aluminium oxide on its surface when exposed to air. This layer sticks strongly to the metal and prevents any further corrosion from occurring.
19) Why do gold ornaments look new even after several years of use?
Ans: Gold ornaments look new even after several years because gold is a highly unreactive metal.
It does not react with air, water, or most chemicals, which means it does not tarnish, rust, or corrode like other metals (e.g., iron or silver). This inherent resistance ensures that its shine and luster remain intact over time.
EXERCISE 7 (C)
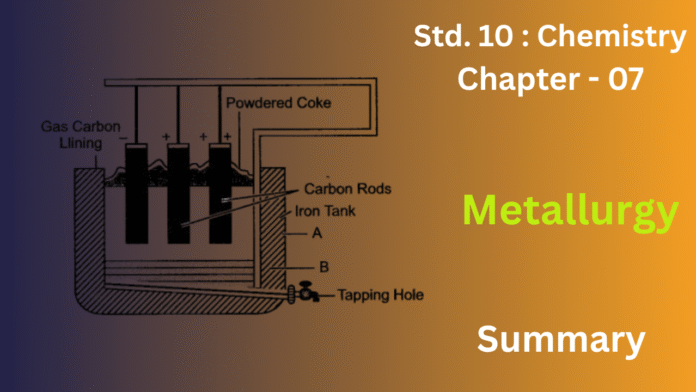
1) Define the term ‘metallurgy’. State the processes involved in metallurgy.
Ans:Metallurgy is the science of working with metals, from extracting them from rocks to making them ready for use.
The key steps in this process are:
- Crushing and Grinding: First, the raw ore is broken down into a fine powder.
- Concentrating the Ore: The valuable mineral is separated from the worthless rocky material, known as gangue.
- Extracting the Metal: This crucial step transforms the ore into a crude metal. It often involves strong heating (calcination or roasting) and a chemical reaction called reduction.
- Refining: The impure, crude metal is then purified to create a high-quality, usable product.
2) Which metal occurs as:
(a) a sulphide
(b) a halide
(c) a carbonate
(d) an oxide
Also give the names of their respective ores
Ans: (a) As a Sulphide
Metal: Zinc
Ore: Zinc Blende (ZnS)
(b) As a Halide
Metal: Silver
Ore: Horn Silver (AgCl)
(c) As a Carbonate
Metal: Calcium
Ore: Limestone (CaCO₃)
(d) As an Oxide
Metal: Aluminium
Ore: Bauxite (Al₂O₃·2H₂O)
3) Distinguish between:
(a) a mineral and an ore,
(b) an ore and a metallic compound
Ans: (a) Mineral vs. Ore
A mineral is a naturally occurring solid with a specific composition and structure. An ore is a rock containing a mineral that is valuable enough to be mined for profit.
Simply put: All ores contain minerals, but only the minerals that can be mined profitably are considered ores.
(b) Ore vs. Metallic Compound
An ore is the natural rock material from which we can economically extract a metal. A metallic compound is the specific chemical substance (like an oxide or sulfide) inside the ore that contains the metal.
In short: The ore is the rock you mine, and it contains the metallic compound you process to get the pure metal.
4) Which metal can be extracted from each one of the following ores.
(a) bauxite (b) calamine (c) haematite
Ans: (a) Bauxite: Aluminium
(b) Calamine: Zinc
(c) Haematite: Iron
5) State three objectives achieved during the roasting of ores
Ans: There are three key objectives achieved during the roasting of ores:
- Removal of Volatile Impurities: It expels moisture, volatile organic matter, and gases like sulfur dioxide (SO₂) from impurities such as arsenic and antimony.
- Conversion of Ores to Oxides: It heats sulphide ores in the presence of air to convert them into metal oxides, which are more suitable for the subsequent reduction process.
- Oxidation of Specific Impurities: It oxidizes and removes certain unwanted impurities, making the ore more porous and easier to handle in the next smelting stage.
6) Give the principles of:
(a) hydrolytic method,
(b) froth floatation
(c) electromagnetic separation
Ans: (a) Hydrolytic Method
Principle: This method separates ore particles based on their differences in density.
Process: The crushed ore is poured over a sloping, vibrating table with grooves (called a Wilfley table) and a stream of water is run over it.
Separation: Heavier (denser) ore particles settle into the grooves and are pushed sideways along the table by the vibrations. Lighter (less dense) gangue particles are washed away by the flowing water.
(b) Froth Floatation
Principle: This method separates sulphide ores from the gangue based on the differences in their wetting properties.
Process: The finely powdered ore is mixed with water in a large tank. Frothing agents (like pine oil) and collectors (which enhance the water-repellency of the ore) are added. Air is blown through the mixture.
Separation: The desired sulphide ore particles, which are repelled by water (hydrophobic), get attached to the air bubbles and rise to the surface to form a froth. The hydrophilic gangue particles sink to the bottom. The froth is then skimmed off.
(c) Electromagnetic Separation
Principle: This method is used to separate magnetic ore particles from non-magnetic impurities (or vice-versa).
Process: The crushed ore is dropped in a thin stream over a roller belt that is moving around a powerful electromagnetic wheel.
Separation: The magnetic particles (like iron ores) are attracted by the magnet and are pulled towards it, falling in a heap closer to the roller. The non-magnetic particles are not attracted and fall straight down due to gravity, forming a separate heap.
7) Name:
(a) the processes involved in
(i) concentration
(ii) refining of ores
(b) two metallic oxides which cannot be reduced by carbon, carbon monoxide or hydrogen
Ans: (a) Name the processes involved in:
(i) The concentration of ores:
This is the first step after mining, where we separate the valuable metal compound from the unwanted earthly materials (gangue). Some common methods are:
- Froth Flotation Process: This is mainly used for sulphide ores. Here, the finely powdered ore is mixed with water and special chemicals. When air is blown through the mixture, the metal sulphide particles attach to the soapy froth and float to the surface, while the gangue sinks to the bottom.
- Magnetic Separation: This method is perfect for ores that are magnetic in nature. For example, if the ore is attracted to a magnet but the gangue is not (or vice-versa), they can be easily separated by passing the powdered ore over a magnetic roller.
- Gravity Separation (Hydraulic Washing): This technique uses the difference in the weight (density) of the ore particles and the gangue. The powdered ore is washed with a stream of water where the heavier ore particles settle down, and the lighter gangue is washed away.
(ii) The refining of metals:
After a metal is extracted from its ore, it often contains impurities. Refining is the process of purifying the crude metal. Some important methods include:
- Electrolytic Refining: This is a very common and effective method, used for metals like copper, zinc, and nickel. Here, a block of the impure metal is made the anode, and a thin sheet of the pure metal is made the cathode. They are placed in an electrolyte solution. When electric current is passed, pure metal from the anode dissolves and gets deposited in a pure form on the cathode, leaving the impurities behind.
- Liquation: This is a simpler method used for metals with a low melting point, like tin. The impure metal is placed on a sloping hearth and heated. The pure metal melts and flows down the slope, separating from the infusible impurities which remain behind.
- Zone Refining: This is a highly sophisticated technique used to produce ultra-pure metals needed for semiconductors and other advanced technologies. A small heated zone is moved slowly along a metal rod. As this zone moves, the impurities dissolve in the molten zone and are carried to one end of the rod, leaving the rest of the rod extremely pure.
(b) Name two metallic oxides which cannot be reduced by carbon, carbon monoxide, or hydrogen.
Some metals have a very strong affinity for oxygen, meaning their oxides are extremely stable. The oxides of such highly reactive metals cannot be reduced by common reducing agents like carbon (C), carbon monoxide (CO), or hydrogen (H₂). Two prominent examples are:
- Aluminium Oxide (Al₂O₃)
- Sodium Oxide (Na₂O)
These metals are so reactive that they hold onto their oxygen atoms very tightly. In fact, these oxides are more stable than the oxides formed by the reducing agents (like CO₂ or H₂O), which is why the reduction reaction is not feasible.
8) Explain the following terms:
(a) flux
(b) gangue
(c) slag
(d) smelting
Ans: (a) Flux
In the process of extracting metals from their ores, a flux is a substance that is added to the furnace to remove unwanted impurities (like sand and earth) known as gangue. The flux reacts chemically with these impurities to form a fusible (easily meltable) material called slag.
Purpose: To remove impurities.
Common Examples: Limestone (CaCO₃) is a common flux used when the impurity is silica (SiO₂).
(b) Gangue
Gangue refers to the unwanted, rocky and earthy materials that are found mixed with the valuable metal mineral in an ore. It is essentially the “waste” or “impurity” that needs to be separated to get the pure metal.
Purpose: It is the impurity that needs to be removed.
Common Examples: Sand (Silica), clay, and other rocky substances.
(c) Slag
Slag is the fusible, waste by-product that is formed when the flux reacts with the gangue during the smelting process. It is lighter than the molten metal and thus floats on top of it, allowing for easy separation.
Purpose: It is the product of the reaction between flux and gangue, making it easy to remove the impurities.
Common Example: When limestone (flux) reacts with silica (gangue), it forms calcium silicate (slag).
(d) Smelting
Smelting is the core chemical process of extracting a metal from its ore by heating it to a very high temperature in a furnace, beyond its melting point. This process involves the action of a reducing agent (like coke or carbon) to convert the metal oxide into the free metal. Smelting is the step where flux is added to remove gangue as slag.
Purpose: To obtain the pure, molten metal.
Common Example: Heating haematite ore (iron oxide) with coke in a blast furnace to produce molten iron.
9)Why does iron or zinc not occur freely in nature?
Ans: Iron and zinc are very reactive, which means they easily mix with elements like oxygen or moisture in the air and soil. This reaction turns them into stable compounds like oxides and carbonates.Because they react so easily, we never find them as pure metals in nature. Instead, they are always found as chemical compounds known as ores. For instance, iron is commonly found as haematite and zinc as zinc blend.
10) What do you observe when hydrogen is passed over heated copper oxide?
Ans: When hydrogen gas is passed over heated black copper oxide, it reduces the compound. This chemical reaction leaves behind brown copper metal and produces water vapor. The steam then condenses into visible water droplets on the cooler surfaces of the test tube.
In short: Hydrogen reduces hot black copper oxide to brown copper and steam, which condenses into water droplets.
11) Compare roasting and calcination
Ans: When hydrogen gas is passed over heated black copper oxide, it reduces the compound. This chemical reaction leaves behind brown copper metal and produces water vapor. The steam then condenses into visible water droplets on the cooler surfaces of the test tube.
In short: Hydrogen reduces hot black copper oxide to brown copper and steam, which condenses into water droplets.
12) (a) Name an ore of zinc.
(b) which process is applied to concentrate it?
(c) How is concentrated ore changed to oxide?
Ans: (a) Name an ore of zinc.
Zinc Blende (also known as Sphalerite)
(b) Which process is applied to concentrate it?
Froth Floatation Process
(c) How is concentrated ore changed to oxide?
By Roasting. It is heated strongly in the presence of excess air.
13) Some metallic oxides can be reduced by hydrogen, carbon and carbon monoxide and some cannot. explain
Ans: A metal oxide can only be reduced by hydrogen, carbon, or carbon monoxide if the metal is less reactive than the reducing agent.
For example:
Reduction is possible for oxides of metals like copper, lead, and iron. Since these metals are below hydrogen/carbon in the reactivity series, they can be displaced.
Reduction is not possible for oxides of highly reactive metals like potassium, sodium, or aluminum. These metals hold onto oxygen too strongly for hydrogen or carbon to remove it.
In simple terms, a substance can only reduce the oxide of a metal that is below it in the reactivity series.
14) How are the following metallic oxides reduced? Write equations:
(a) Iron (II) oxide,
(b) Zinc oxide
Ans: Metallic oxides are reduced to their metals by heating them with a suitable reducing agent like carbon or carbon monoxide.
(a) Iron(II) oxide
It is reduced by heating with Carbon Monoxide (CO).
Equation:
FeO + CO → Fe + CO₂
(b) Zinc oxide
It is reduced by heating with Carbon (C).
Equation:
2ZnO + C → 2Zn + CO₂
15) State why aluminium is extracted from its oxide by electrolysis while copper, lead,
Ans: iron by reducing agents and mercury and silver by thermal decomposition.
The extraction method depends on the reactivity of the metal.Aluminium is more reactive than carbon. Therefore, carbon cannot remove oxygen from its oxide. A stronger method, electrolysis, is needed to force the reaction.Copper, Lead, Iron are less reactive than aluminium but more reactive than carbon. They can be extracted by heating their oxides with carbon (a reducing agent), which takes away the oxygen.
Mercury and Silver are very unreactive. Their oxides are unstable and break down easily when heated, a process called thermal decomposition. No extra reducing agent is needed.
16) An ore being heated in air forms sulphurous anhydride. Write the process used for the concentration of this ore.
Ans: The process used for the concentration of this ore is Froth Flotation.This method is used for sulphide ores. Since the ore produces sulphurous anhydride (SO₂) on heating, it confirms it is a sulphide ore, making froth flotation the suitable concentration process.
17) (a) on which factors does purification of metals depend? (
b) name the methods used for purification
(c) How is electro-refining done?
Ans: (a) Factors affecting the purification of metals:
The choice of purification method mainly depends on:
The nature of the metal.
The type and amount of impurities present.
The intended use of the pure metal.
(b) Methods used for purification:
Common methods include:
Electrolytic Refining (Electro-refining)
Distillation
Liquation
Zone Refining
(c) How electro-refining is done:
In electro-refining:
A thick block of the impure metal is made the anode.
A thin strip of the pure metal is made of the cathode.
A water-soluble salt solution of the same metal is used as the electrolyte.
When electric current is passed, the impure metal from the anode dissolves into the electrolyte.
The pure metal from the electrolyte is deposited on the cathode.
Impurities settle down below the anode as anode mud.
18)
(a) Ag2O —>…………………….
(b) MnO2 + 4Al —> …………….
(c) Cu (OH)2 —> ………………..
(d) ZnCO3 —> ………………….
(e) 2NaNO3 —> …………………
(f) 2Pb(NO3)2 —> ………………
(g) 2AgNO3 —> ………………..
(h) 2Cu2O +Cu2S —> …………..
(i) HgS + O2 —> ………………
Ans: Balanced equations are:
(a) Ag2O —> 4Ag +O2
(b) MnO2 + 4Al —> 3Mn +2Al2O3 + heat
(c) Cu (OH)2 —> CuO + H2O
(d) ZnCO3 —> ZnO + CO2
(e) 2NaNO3 —> 2NaNO2 + O2
(f) 2Pb(NO3)2 —> 2PbO +4NO2 +O2
(g) 2AgNO3 —> 2Ag + 2NO2 +O2
(h) 2Cu2O +Cu2S —> 6Cu + SO2
(i) HgS + O2 —> Hg +SO
EXERCISE .7 (D)
1) State the position of aluminium in the periodic table.
Ans: Position of Aluminium in the Periodic Table
Aluminium is located in Period 3 and Group 13 of the modern periodic table.
To break this down a bit more:
Group 13: This is the vertical column in which aluminium resides. Elements in this group have three electrons in their outermost shell, which heavily influences their chemical properties. Aluminium is the second element in this group, following boron.
Period 3: This is the horizontal row. Being in the third period means that aluminium has three electron shells surrounding its nucleus.
In summary, you can find aluminium sitting in the box that is the third one down and the thirteenth one across (if you start counting from the left, excluding the transition metals block). It is a p-block element, positioned to the left of silicon and below boron.
2) Give the chemical names and formulae of any three ores of aluminium.
Ans: 1)Bauxite
This is the primary ore used for producing aluminium.It is not a pure mineral but a mixture, mainly containing hydrated aluminium oxide along with impurities like iron oxide.
2)Cryolite
Its chemical name is Sodium Aluminium Fluoride (Na₃AlF₆).Its main purpose is to dissolve aluminium oxide, which significantly lowers the energy required for the electrolytic extraction of aluminium.
3)Corundum
This is the crystalline and pure form of aluminium oxide (Al₂O₃).It is an extremely hard mineral. When it contains trace amounts of other elements, it forms precious gemstones such as rubies (red) and sapphires (blue).
3) Which impurities are present in bauxite.
Ans: The most significant impurities found in bauxite are:
1. Iron Oxides (like Hematite)
This is the impurity that gives most bauxite its characteristic rusty red or brown color. Think of it as the “rust” within the ore. While it’s harmless in terms of the chemical process, its presence is a major visual marker. In large quantities, it represents a portion of the ore that doesn’t yield any aluminum, making the processing less efficient.
2. Silica
Silica is a major troublemaker in the aluminum production process. It doesn’t just sit idly by; it reacts chemically with the other components. During refining, silica can dissolve and then precipitate, forming a compound that causes significant losses of the valuable aluminum-containing chemicals. This not only reduces the yield but also creates scaling in equipment, leading to downtime and maintenance costs. It’s often found as quartz sand or within clay minerals like kaolinite.
3. Titanium Dioxide
Typically present as the mineral rutile, titanium dioxide is a stubborn impurity. It is largely inert and doesn’t react much during the chemical extraction process. Because of this, it doesn’t cause the same kind of reactive losses as silica, but it simply dilutes the ore and ends up as a solid waste product. This waste, known as “red mud,” is a major byproduct of aluminum production that requires careful management.
4. Other Minor Impurities
Bauxite can also contain a whole suite of other elements in smaller amounts. These can include silicates of various forms, phosphates, and even traces of zircon. While they are present in lesser quantities, some can have specific impacts, such as interfering with the efficiency of the chemical process or ending up in the final product if not carefully controlled.
In essence, the industrial process of turning bauxite into aluminum is largely a story of separation—designing methods to systematically isolate the aluminum from these common and persistent impurities.
4) What is red mud, how is it removed?
Ans: Red mud is a toxic, alkaline waste product created during the Bayer process for refining bauxite into alumina (the raw material for aluminum).It is a slurry with a characteristic red color due to its high iron oxide content.
How it is removed:
Since it is a waste product, it is not “removed” in a cleaning sense but is disposed of or stored. The primary method is:
Pumping it into large, specially designed containment ponds or reservoirs called tailings dams. Here, the solid mud settles, and the water is often treated and recycled.Due to its environmental risks, there is significant research into recycling methods to extract valuable metals (like iron, titanium, and rare earth elements) from the mud or to use it in building materials, but these are not yet widely implemented.
5) Why is electrolytic reduction done to obtain aluminium?
Ans:Electrolytic reduction is used to obtain aluminium because:
- Aluminium is highly reactive – It has a strong affinity for oxygen, so it cannot be extracted by common reducing agents like carbon.
- Oxide is very stable – Aluminium oxide (Al₂O₃) is a very stable compound. It does not decompose easily on heating.
- Requires a strong method – The strong bond between aluminium and oxygen in its oxide can only be broken by using a powerful method like electrolysis.
Therefore, electrolytic reduction (using a strong electric current) is the only commercially viable method to extract aluminium from its oxide.
6) Give the ionization reactions of electrolyte used in Hall’s process. write the reaction at the cathode and the anode. Why the anode has to be replaced in this process?
Ans: Electrolytic reduction is used to obtain aluminium because aluminium is highly reactive. It has a strong affinity for oxygen, so it cannot be reduced by common reducing agents like carbon. The high stability of aluminium oxide (Al₂O₃) requires a very strong method for its extraction.Therefore, electrolysis of a molten mixture of alumina and cryolite is employed. This process provides the necessary strong driving force to break the aluminium-oxygen bonds and produce pure aluminium metal.
7) (a) Name the process by which the refining of aluminium is done.
(b) Where are the cathode and anode in the electrolytic cell? Name the material used for these?
(c) state the reactions at the two electrodes.
Ans: (a) Hoope’s Process
Aluminium refining is done by Hoope’s process, which uses electrolysis to obtain high-purity aluminium.
(b) Electrodes in Hooper’s Cell
Cathode: Located at the bottom, made of graphite lining.
Anode: Placed at the top, made of graphite rods.
(c) Electrode Reactions
Cathode (Reduction):
Al3++3e−→Al (l)Al 3++3e − →Al (l)
(Aluminium ions gain electrons to form pure molten aluminium)
Anode (Oxidation):
C (s)+O2−→CO (g)+2e−C (s)+O 2− →CO (g)+2e −
(Oxide ions lose electrons, reacting with carbon to form carbon monoxide gas)
8) How does aluminum react with the following:
(a) Air, (b) Water, (c) Acid, (d) Base
Ans:(a) Reaction with Air
Aluminum reacts rapidly with oxygen in the air, forming a thin, hard layer of aluminum oxide (Al₂O₃) on its surface. This layer is highly stable and prevents further corrosion, making aluminum highly resistant to weathering.
(b) Reaction with Water
Under normal conditions, aluminum does not react noticeably with water. This is because the protective aluminum oxide layer on its surface is insoluble in water and shields the metal underneath from further reaction.
(c) Reaction with Acid
Aluminum reacts with acids like hydrochloric acid (HCl) to produce hydrogen gas and the corresponding salt. The protective oxide layer is dissolved by the acid, allowing the reaction to proceed. For example:
2Al + 6HCl → 2AlCl₃ + 3H₂
(d) Reaction with Base
Aluminum reacts vigorously with strong bases like sodium hydroxide (NaOH). The oxide layer is dissolved, and the metal reacts to form hydrogen gas and a soluble salt called sodium aluminate. For example:
2Al + 2NaOH + 6H₂O → 2NaAl(OH)₄ + 3H₂
9) What is the role of cryolite (Na3AIF6) in the electrolytic reduction of alumina in Hall’s process?
Ans: (a) Reaction with Air
Aluminum reacts rapidly with oxygen in the air, forming a thin, hard layer of aluminum oxide (Al₂O₃) on its surface. This layer is highly stable and prevents further corrosion, making aluminum highly resistant to weathering.
(b) Reaction with Water
Under normal conditions, aluminum does not react noticeably with water. This is because the protective aluminum oxide layer on its surface is insoluble in water and shields the metal underneath from further reaction.
(c) Reaction with Acid
Aluminum reacts with acids like hydrochloric acid (HCl) to produce hydrogen gas and the corresponding salt. The protective oxide layer is dissolved by the acid, allowing the reaction to proceed. For example:
2Al + 6HCl → 2AlCl₃ + 3H₂
(d) Reaction with Base
Aluminum reacts vigorously with strong bases like sodium hydroxide (NaOH). The oxide layer is dissolved, and the metal reacts to form hydrogen gas and a soluble salt called sodium aluminate. For example:
2Al + 2NaOH + 6H₂O → 2NaAl(OH)₄ + 3H₂
In short, aluminum’s behavior is dominated by its protective oxide layer, which makes it passive in air and water but reactive with both acids and bases.
10) (a) Aluminium is a more active metal than iron, but suffers less corrosion. Why?
(b) Explain and give reasons why aluminium vessels should not be cleaned with powders containing alkalis.
Ans: (a) Aluminium is more active than iron but suffers less corrosion because:
Aluminium reacts rapidly with oxygen in the air, forming a thin, strong, and adherent layer of aluminium oxide (Al₂O₃) on its surface. This oxide layer is non-porous and highly stable. It acts as a protective shield, preventing further contact between the underlying metal and environmental agents like oxygen and moisture. In contrast, the rust (hydrated iron oxide) formed on iron is porous, flaky, and brittle. It peels off easily, continuously exposing fresh iron surface to further corrosion.
(b) Aluminium vessels should not be cleaned with alkali-containing powders because:
Alkalis (like sodium hydroxide or sodium carbonate found in cleaning powders) react chemically with the protective aluminium oxide layer. They dissolve this layer, destroying the protective shield. Once this layer is removed, the alkalis then attack the bare aluminium metal directly, corroding it and producing hydrogen gas. This reaction damages the vessel, leading to pitting and a shortened lifespan.
11) (a) Give the name and formula of the main ores of iron and zinc
(b) How is the main ore of aluminium concentrated?
(c) Why ‘the food containing iron salts’ should not be cooked in aluminium utensils?
Ans: (a) Ores of Iron and Zinc
Iron:
Name: Haematite
Formula: Fe₂O₃
Zinc:
Name: Zinc Blende (or Sphalerite)
Formula: ZnS
(b) Concentration of Aluminium Ore (Bauxite)
The main ore of aluminium, bauxite (Al₂O₃.xH₂O), is concentrated by Baeyer’s Process. In this method, the crushed ore is heated with a concentrated solution of sodium hydroxide. The aluminium oxide dissolves to form sodium aluminate, while impurities like iron oxide and silica remain undissolved as red mud. The insoluble impurities are filtered out, and pure aluminium hydroxide is precipitated from the filtrate.
(c) Reason for not cooking iron-rich food in aluminium utensils
Food containing iron salts should not be cooked in aluminium utensils because aluminium is more reactive than iron. A displacement reaction occurs where aluminium ions from the utensil go into the food, and iron ions from the food get deposited. This leads to two problems:
The aluminium utensil gets corroded (pitted).
The food gets contaminated with aluminium salts, which can be harmful to health.
12) Explain with reasons:
(a) In the electrolytic reduction of alumina, the graphite anode is gradually consumed.
(b) Roasting is carried out on sulphide ores and not on carbonate ores.
(c) Carbon can reduce lead oxide but not aluminium oxide
Ans: (a) In the electrolytic reduction of alumina, the graphite anode is gradually consumed.During the electrolysis of alumina, oxygen gas is produced at the anode.
The high temperature of the electrolytic cell causes this oxygen to react with the graphite (carbon) of the anode, forming carbon dioxide gas.
This continuous chemical reaction:
C (graphite)+O2→CO2C (graphite)+O 2 →CO2
slowly burns away the anode, leading to its gradual consumption.
(b) Roasting is carried out on sulphide ores and not on carbonate ores.
Roasting is a process specifically for sulphide ores because it involves heating the ore strongly in the presence of air to convert the sulphide into the metal oxide and sulphur dioxide gas.
Carbonate ores, on the other hand, are converted to their metal oxides simply by calcination (heating strongly in the absence of air), which drives off carbon dioxide gas. Applying roasting (with air) to a carbonate ore is unnecessary and does not aid the process.
(c) Carbon can reduce lead oxide but not aluminium oxide.
This is based on the relative reactivity of metals.
Aluminium is higher than carbon in the reactivity series, meaning aluminium has a stronger tendency to hold onto oxygen. Therefore, carbon cannot remove oxygen from aluminium oxide.
Lead, however, is lower than carbon in the reactivity series. This means carbon can successfully remove oxygen from lead oxide, acting as a reducing agent.
13) (a) Why is flux used in the blast furnace?
(b) what does it form with silica present in the ore?
(c) How is it removed?
Ans: (a) Flux removes impurities from iron ore.
Iron ore, such as haematite, is never found in a pure state. It is naturally mixed with rocky, earthy materials known as gangue. The most common and troublesome component of this gangue is silica (silicon dioxide, or SiO₂), which is essentially sand. If these impurities were left in the mix, they would make the final iron product brittle and unusable. The flux, which is typically a basic substance like limestone (calcium carbonate, CaCO₃), is added to the blast furnace along with the iron ore and coke. Its primary job is to chemically target and remove these unwanted impurities.
(b) It reacts with silica to form a slag.
This is where the key chemical reaction happens. Inside the intense heat of the blast furnace, the limestone first breaks down into calcium oxide and carbon dioxide:
CaCO₃ (limestone) → CaO (calcium oxide) + CO₂ (carbon dioxide)
The newly formed calcium oxide (CaO) is highly reactive. It then seeks out the acidic silica (SiO₂) from the gangue and combines with it in a spectacular chemical dance. This reaction produces a completely new substance called calcium silicate:
CaO (calcium oxide) + SiO₂ (silica) → CaSiO₃ (calcium silicate)
This calcium silicate is what we know as slag.
(c) This slag is lighter, floats on the molten iron, and is removed separately.
The formation of slag solves a major physical problem as well as a chemical one. Slag has a lower density than the molten iron. In the fiery, liquid environment at the bottom of the blast furnace, the two liquids separate based on their weight, much like oil and water. The heavier, denser molten iron (called pig iron) sinks to the very bottom of the furnace. Meanwhile, the lighter, molten slag floats on top, forming a distinct layer that covers the iron.
This separation is crucial. At the end of the process, the blast furnace is tapped. There are separate tap holes at different heights. The lower tap hole is for the molten iron to be drained away for further processing or casting. The upper tap hole allows the slag to be removed separately. This ensures that the pure iron is not contaminated by the waste material.
In summary, the flux transforms solid, infusible impurities into a liquid slag that can be easily separated, protecting the iron and allowing for the efficient production of pure metal. The slag itself is not just waste; it is often cooled and used in various applications like road construction and cement manufacturing.
14) Name an ore which is concentrated by:
(a) forth floatation process,
(b) magnetic separation
Ans: (a) Froth Flotation Process
An ore that is concentrated using the froth flotation process is Zinc Blende (also known as Sphalerite). Its chemical formula is ZnS (Zinc Sulphide).
Explanation:
The froth flotation process is particularly effective for separating sulphide ores from their earthy impurities, called gangue. Zinc Blende is a sulphide ore. In this process, the finely crushed ore is mixed with water and specific chemicals.Collectors (like pine oil) are added, which attach to the particles of the sulphide ore, making them water-repellent (hydrophobic).Frothers are added to produce a stable froth.Air is blown through the mixture, causing the hydrophobic ore particles to be carried to the surface with the froth, which is then skimmed off.The hydrophilic gangue particles, which wetted by water, sink to the bottom.This method efficiently enriches the percentage of Zinc in the ore by removing a large portion of the unwanted silica and other impurities.
(b) Magnetic Separation
An ore that is concentrated using magnetic separation is Magnetite. Its chemical formula is Fe₃O₄.
Explanation:
Magnetic separation is used when one component of the ore mixture is magnetic and the other (the gangue) is non-magnetic. Magnetite, as the name suggests, is a naturally magnetic mineral of iron. In this process, the crushed ore is passed over a rapidly moving belt that is wound around a roller containing an electromagnet. The magnetic particles of Magnetite are attracted by the magnet and stick to the belt. They are carried a little further and then thrown away from the belt due to the lack of magnetic influence at the end. The non-magnetic gangue particles are not attracted and fall off the belt in a separate heap. This effectively separates the valuable iron ore from the waste material.
15) Distinguish between electrolytic methods of reduction and refining.
Ans: Electrolytic Reduction
Purpose: To extract a pure metal from its fused (molten) ore or compound.
What it does: It is a process of winning the metal. It converts metal ions (e.g., Al³⁺, Na⁺) from their compound into their neutral, metallic state.
Key Feature: The impure metal ore itself acts as the cathode, where reduction (gain of electrons) happens.
Example: Extracting aluminium from molten alumina (Al₂O₃) using the Hall-Héroult process.
Electrolytic Refining
Purpose: To purify a metal that is already in its impure metallic form.
What it does: It is a process of purifying an already obtained metal.
Key Feature: A thick block of the impure metal acts as the anode. Pure metal is deposited on a thin pure metal cathode. Impurities settle as “anode mud.”
Example: Refining copper, where impure copper anodes are used to produce 99.99% pure copper cathodes.
16) Give three ways in which the metal zinc differs from the non-metals carbon. At least one of the differences must be a chemical difference.
Ans: Here are three key ways zinc differs from carbon:
- Metal vs. Non-Metal: Zinc is a metal, making it shiny and malleable. Carbon is a non-metal, with forms like graphite being dark and brittle.
- Reaction with Acid: Zinc reacts with dilute acids, fizzing and producing hydrogen gas. Carbon shows no reaction in the same situation.
- Electrical Conductivity: Zinc is a consistent conductor of electricity. Carbon’s conductivity varies; graphite conducts well, while diamond is an excellent insulator.
EXERCISE. 7 (E)
1) State a reason why zinc is used in:
(a) galvanization,
(b) dry cells
(c) cosmetics?
Ans:(a) Galvanization
Zinc is used for galvanization primarily because it acts as a sacrificial protector. When a layer of zinc is coated onto iron or steel, it reacts more readily with the environment (like air and moisture) than the iron does. So, even if the coating gets scratched, the zinc will “sacrifice” itself and corrode first, leaving the underlying iron intact and preventing rust.
(b) Dry Cells
In dry cells (like the common AA or D batteries), zinc is used to make the outer casing, which acts as the negative terminal (anode). It’s an excellent choice because it’s a relatively reactive metal that can efficiently generate electrical energy through chemical reactions inside the cell. Furthermore, zinc is inexpensive, can be easily molded into the required can-like shape, and is safe for this purpose.
(c) Cosmetics
Zinc is a common ingredient in cosmetics, especially in products like sunscreens and baby lotions, because of its compound Zinc Oxide. This compound is highly effective at blocking harmful ultraviolet (UV) rays from the sun, acting as a physical sunscreen. It is also known for its soothing and anti-inflammatory properties, which helps calm skin irritations like diaper rash or minor redness.
2) State on what special properties the use of each of these metals depends:
(a) aluminium
(b) zinc
Ans: (a) Aluminium
The use of aluminium depends on its unique combination of:
Low density (it is very lightweight).
Resistance to corrosion due to a protective oxide layer that forms on its surface.
Good electrical and thermal conductivity.
(b) Zinc
The use of zinc depends primarily on two key properties:
Excellent corrosion resistance, as it forms a protective patina and is widely used to galvanize steel, where it sacrificially protects the iron from rusting.
Low melting point, which makes it ideal for use in die-casting into shapes and for hot-dip galvanizing.
3) Explain the following:
(a) zinc is used to cover iron so as to prevent rusting of iron why?
(b) A neutral gas other than oxygen which is formed at the anode during electrolysis of fused alumina
(c) Nitric acid can be stored in aluminium containers.
Ans:
(a) Zinc is used to cover iron to prevent rusting because:
Zinc is more reactive than iron. When zinc coating is scratched, it still protects the iron underneath by acting as a sacrificial anode. The zinc loses electrons and corrodes first, while the iron does not. This process is known as cathodic protection. Additionally, zinc forms a protective layer of zinc carbonate on its surface, which prevents further corrosion.
(b) A neutral gas other than oxygen formed at the anode during electrolysis of fused alumina:
The gas is Carbon monoxide (CO).
Reason: The anode is made of graphite (carbon). During electrolysis, the oxygen gas produced at the anode reacts with the hot carbon anode to form carbon monoxide.
2C (anode) + O₂ → 2CO
(c) It seems strange, but strong, concentrated nitric acid can be safely stored in aluminium containers because of a special chemical trick called passivation.When the concentrated acid touches the aluminium, it doesn’t eat through the metal. Instead, it causes the aluminium surface to instantly form an ultra-thin, invisible, and very tough layer of aluminium oxide.This oxide layer acts like a perfect, non-porous shield. It sticks tightly to the metal, protecting the reactive aluminium underneath from any further contact with the acid. In effect, the acid creates its own protective barrier.This only works with concentrated nitric acid. Dilute nitric acid reacts differently and will corrode the aluminium.
4) State the use of: (a) cast iron (b) wrought Iron (c) Mild steel, (d) hard steel.
Ans: (a) Cast Iron
Used for machine parts, pipes, and engine blocks due to its hardness, brittleness, and low-cost casting of complex shapes.
(b) Wrought Iron
Ideal for chains, crane hooks, and gates because it is tough, ductile, and resists corrosion well.
(c) Mild Steel
Common in structural beams, car bodies, and domestic items as it is soft, ductile, and easily welded.
(d) Hard Steel
Suitable for cutting tools, drills, and blades owing to its high hardness, strength, and ability to hold a sharp edge.
5) Which metal is used for:
(a) making pipes, buckets, water tanks,
(b) lithographic plates for printing
(c) making face creams
Ans: (a) Making pipes, buckets, water tanks:
Galvanized Iron (GI) or Zinc-coated iron is commonly used.
(b) Lithographic plates for printing:
Zinc or sometimes Aluminum is used for lithographic plates.
(c) Making face creams:
Zinc oxide is a common metal compound used in face creams for its soothing and protective properties.
6) Give reasons, why aluminum is used in:
(a) making alloys
(b) wrapping chocolates
(c) painting electric and telegraphic poles
(d) In aluminiothermy
(e) In making ships
Ans: (a) Making Alloys
Aluminum is soft by itself. When alloyed with metals like copper or magnesium, it becomes stronger, harder, and more durable while staying lightweight. These improved properties make aluminum alloys ideal for construction, aircraft, and automobiles.
(b) Wrapping Chocolates
Aluminum foil wraps chocolates effectively because it blocks light, moisture, and air. This keeps the chocolates fresh, preserves their flavor and aroma, and reduces the risk of melting.
(c) Painting Electric and Telegraphic Poles
A special aluminum paint is used on poles because it resists corrosion well. The metallic layer reflects heat and light, protecting wooden poles from weathering and extending their life.
(d) In Aluminothermy
Aluminum acts as a strong reducing agent in aluminothermy. It extracts oxygen from metal oxides (such as iron oxide), releasing intense heat. This process is used for welding railway tracks by reducing other metals to a molten state.
(e) In Making Ships
Aluminum alloys are used in shipbuilding because they are lightweight and resist corrosion from seawater. This reduces the ship’s weight, improving fuel efficiency, speed, and payload capacity.
7) Aluminum is used in thermite welding:
(a) what is a thermit?
(b) what is the ignition mixture?
(c) write reaction for process?
Ans: a) What is thermit?
Thermit (or thermite) is a mixture of a metal powder and a metal oxide powder. For welding iron, it is typically a combination of aluminum powder and iron(III) oxide (rust) powder.
(b) What is the ignition mixture?
The ignition mixture is a highly flammable substance used to start the thermite reaction. Since the thermite reaction requires very high heat to begin, a common ignition mixture is magnesium ribbon, which burns at an extremely high temperature to provide the necessary activation energy.
(c) Write a reaction for the process.
The chemical reaction for the thermite process using aluminum and iron oxide is:
Fe₂O₃ + 2Al → 2Fe + Al₂O₃ + Heat
(Iron Oxide + Aluminum → Iron + Aluminum Oxide + Heat)
In this reaction, aluminum reduces the iron oxide, producing molten iron used for welding and aluminum oxide as a slag.
8) What is an alloy? How do the properties of an alloy differ from its constituents?
Ans:
- Alloys are made by mixing metals to create a superior material. Their key advantages over pure metals include:
- Increased Strength: Alloys like steel are significantly stronger than their base metals.
- Lower Melting Points: Alloys such as solder melt more easily, making them simpler to use.
- Enhanced Corrosion Resistance: Materials like stainless steel resist rust and wear far better than pure iron.
- Better Casting and Appearance: Some alloys fill molds more effectively or have a more attractive look, like the color and shine of brass.
9) Name three alloys of steel. Give their compositions and uses.
Ans:
| Alloy’s name | Composition | Uses |
| 1. Stainless steel 2. Manganese steel 3. Tungsten steel | 73% Fe,18%Cr,8%Ni,1%C 85% Fe,1%C ,14%Mn 84%Fe, 5%W, 1%C | Used for making utensils, cutlery, ornamental pieces and surgical instruments. Used for making rock drills and armour plates. Used for cutting tools for high speed lathes. |
10) Both brass and bronze contain copper as major constituents Name other elements in these alloys.
Ans: Brass:
The other main element is zinc.
Bronze:
The other main element is tin.
11) Name an alloy of:
(a) aluminium used in aircraft construction
(b) lead used in electrical wiring or electrical work in joining metals.
(c) copper in electrical appliances or household vessels
(d) zinc used in simple voltaic cells
Ans: (a) Duralumin
(b) Solder
(c) Brass
(d) Brass
12) What is an amalgam? State its use with an example.
Ans: An amalgam is a mixture where mercury is combined with other metals.Its most well-known use is in dentistry for fillings. A common “silver filling” is made by blending liquid mercury with a powdered mix of silver, tin, and copper to form a durable plug for a cavity.
13) (a) state two properties of brass that render it more useful for some purpose than its components
(b) a metal which forms a liquid alloy at ordinary temperature
Ans: (a) Two properties of brass that make it more useful than its components:
Higher Strength and Hardness: Brass is stronger and harder than pure copper or pure zinc, making it more durable for applications like plumbing fittings and musical instruments.
Better Machinability: Brass is much easier to shape and cut (more malleable) than its components, which is ideal for creating detailed components like gears and locks.
(b) A metal which forms a liquid alloy at ordinary temperature:
Mercury (It forms liquid alloys with other metals, called amalgams, at room temperature).
14) What is magnalium? Name the main elements present in it? Write its one use.
Ans: Magnalium is a strong and lightweight alloy primarily composed of magnesium and aluminium. Due to its favourable properties, it is commonly used to manufacture scientific instruments and lightweight aircraft components.
15) Name the constituents of: (a) Duralumin (b) solder, (c) Bronze (d) Invar
Ans: (a) Duralumin
Duralumin is a strong, lightweight alloy where aluminum acts as the primary metal. It’s made by combining aluminum with copper, and then adding small but important quantities of magnesium and manganese. The addition of copper is the key to its strength; it allows the alloy to be heat-treated to a hardness similar to steel, while remaining very light. This combination makes it a favorite in the aerospace industry for building aircraft frames and other structures where weight and strength are critical.
(b) Solder
Solder is a metal alloy used to create a permanent bond between metal parts, most famously in electronics and plumbing. The classic, traditional formula for solder is a mixture of lead and tin. However, due to the health and environmental risks of lead, modern lead-free solders have become standard. These new solders typically use tin as the main component, with a small amount of copper mixed in to improve its properties and prevent corrosion.
(c) Bronze
Bronze is one of the oldest known alloys, with a history stretching back thousands of years. It is primarily composed of copper, which is alloyed with tin. The proportion of tin can vary, which changes the bronze’s characteristics, but it generally creates a metal that is harder and more durable than copper on its own. Historically, this hardness made it invaluable for tools, weapons, and sculptures. It’s also known for its distinctive reddish-gold color and its resistance to corrosion.
(d) Invar
Invar is a very unique nickel-iron alloy. Its defining characteristic is an exceptionally low rate of thermal expansion, meaning it barely expands or contracts when exposed to changes in temperature. This special property comes from its specific composition, which is approximately 64% iron and 36% nickel. Because it maintains its shape and size so well under temperature fluctuations, it is incredibly useful in precision instruments like pendulum clocks, scientific measuring devices, and large-scale structures that are exposed to varying weather conditions.
MISCELLANEOUS EXERCISE
1) For each substance listed below, explain its significance in the extraction of aluminium. (a) bauxite (b) Sodium hydroxide (c) Cryolite (d) Graphite
Ans: (a) Bauxite
The principal ore of aluminium, processed to obtain alumina (aluminium oxide).
(b) Sodium Hydroxide
Used in bauxite purification to dissolve alumina, forming soluble sodium aluminate and removing impurities.
(c) Cryolite
Lowers the melting point of alumina from ~2000°C to 950°C, making electrolysis more energy efficient.
(d) Graphite
Acts as electrodes in the electrolytic cell. The anode oxidizes, forming CO₂ and eroding over time.
2) From the metals: copper iron, magnesium, sodium and zinc, select a different metal in each case which:
(a) does not react with dilute hydrochloric acid
(b) can form 2 + and 3 + ions
(c) has a hydroxide that reacts with both acids and alkalis
(d) for not reacting with cold water but reacts with steam when heated.
Ans: (a) Does not react with dilute hydrochloric acid
→ Copper (It is below hydrogen in the reactivity series.)
(b) Can form 2+ and 3+ ions
→ Iron (Forms Fe²⁺ and Fe³⁺ ions.)
(c) Has a hydroxide that reacts with both acids and alkalis
→ Zinc (Zinc hydroxide is amphoteric.)
(d) Does not react with cold water but reacts with steam when heated
→ Magnesium (Reacts slowly with steam but not with cold water under normal conditions.)
3) Arrange the metals in (2) in the decreasing order of reactivity.
Ans: Arrangement of metal in decreasing order of reactivity are:
Sodium > Magnesium > Zinc > Iron > copper
4) In order to obtain 1 tonne of aluminium the following inputs are required. 4 tonnes of bauxite, 150 kg of sodium hydroxide and 600 kg of graphite. The aluminium compound in bauxite is aluminium oxide and the main impurity is iron (III) oxide, Aluminium is obtained by the electrolysis of aluminium oxide dissolved in cryolite
(a) when bauxite is treated with sodium hydroxide solution, what happens to (i) the aluminium oxide (ii) the iron (III) oxide
(b) (i) Name the process used for the purification of bauxite (ii) Write the equation for the action of heat on aluminium hydroxide
(c) (i) write the formulae of cryolite (ii) Write down the word which correctly completes the following sentences. By dissolving aluminium oxide in cryolite a ………. (conducting/non conducting) solution is produced. (iii) why is so much graphic required for the electrolytic process? (iv) Write the equation for the reaction which takes place at cathode.
(d) In construction work, why is the alloy of aluminium duralumin used rather than pure aluminium?
Ans: (a) Reaction with NaOH
(i) Al₂O₃ dissolves to form sodium aluminate.
(ii) Fe₂O₃ remains insoluble and is removed by filtration.
(b) Purification & Heating
(i) Bayer process.
(ii) 2Al(OH)₃ → Al₂O₃ + 3H₂O
(c) Electrolytic Process
(i) Cryolite: Na₃AlF₆
(ii) Lowers melting point and conducts electricity.
(iii) Graphite anodes burn in oxygen, needing frequent replacement.
(iv) Cathode: Al³⁺ + 3e⁻ → Al(l)
(d) Duralumin vs Pure Aluminium
Duralumin is stronger and harder, making it more suitable for construction than soft pure aluminium.
5) Aluminium is extracted from its chief ore, bauxite. The ore is first purified and then the metal is extracted from it by electrolytic reduction
(a) Write three balanced equation for the purification of bauxite
(b) Name a chemical used for dissolving aluminium oxide, In which state of subdivision is the chemical used?
(c) Write an equation for the reaction which takes place at the anode during the extraction of aluminium by the electrolytic process.
(d) Mention one reason for the use of aluminium in thermite welding.
Ans: (a) Purification of bauxite:
Al₂O₃ + 2NaOH → 2NaAlO₂ + H₂O
NaAlO₂ + 2H₂O → NaOH + Al(OH)₃
2Al(OH)₃ → Al₂O₃ + 3H₂O (on heating)
(b) Dissolving aluminium oxide:
The chemical used is cryolite (Na₃AlF₆). It is used in the molten (fused) state.
(c) Anode reaction during extraction:
The primary reaction is:
2O²⁻ → O₂ + 4e⁻
The oxygen gas produced then reacts with the carbon anode, forming carbon dioxide (CO₂).
(d) Use in thermite welding:
Aluminium is used because it is a powerful reducing agent. It can easily displace less reactive metals from their oxides (like iron from Fe₂O₃), and this reaction is highly exothermic, releasing a massive amount of heat needed to melt the metal for welding.
Question 1(2005):
(a) A to F below relate to the source and extraction of either zinc or aluminium:
A. Bauxite
B. Coke
C. Cryolite
D. Froth floatation
E. Sodium hydroxide solution,
F. Zinc blende (i) Write down the three letters each from the above list which are relevant to: 1. Zinc 2. Aluminum
(ii) Fill in the blanks using the most appropriate words from A to F.
1. The ore from which aluminum is extracted must first be treated with …………. So that pure aluminum oxide can be obtained.
Ans: (a)(i)
Zinc → B (Coke), D (Froth floatation), F (Zinc blende)
Aluminium → A (Bauxite), C (Cryolite), E (Sodium hydroxide solution)
(a)(ii)
The ore from which aluminum is extracted must first be treated with E (Sodium hydroxide solution so that pure aluminum oxide can be obtained.
Question 2(2005): Calcium copper, lead aluminium zinc chromium, magnesium and iron. Choose the major metals from the list given above to make the following alloys: (a) Stainless steel (b) brass
Ans: (a) Stainless steel
Iron and Chromium.
(b) Brass
Copper and Zinc.
Question (2006): Name the following:
(a) A metal which is liquid at room temperature
(b) The process of heating an ore to a high temperature in the presence of air.
(c) The compound formed by the reaction between calcium oxide and silica
(d) A compound which is added to lower the fusion temperature of the electrolytic bath in the extraction of aluminium.
(e) Name an allotrope of a non-metal that allows electricity to pass through it.
Ans: (a) Mercury
(b) Roasting
(c) CaSiO3
(d) Cryolite
(e) Graphite
Question 1(2007): From the list of characteristics given below, select the five which are relevant to non-metals and their compounds:
A. ductile
B. Conduct electricity
C. Brittle
D. Acidic oxide
E. Basic oxides
F. Discharge at anode
G. Discharge at cathode
H. Ionic chlorides
I. Covalent chlorides
J. Reaction with dilute sulphuric acid yields hydrogen,
K. 1, 2, or 3 valence electrons
L. 5, 6, 7 valence electrons (write the five letters corresponding to the correct characteristics)
Ans: Acidic oxide(D)
Discharged at anode (F)
Covalent chlorides (I)
5,6,7 valence electrons (L)
Brittle(C)
Question 2(2007): The following is an extract from ‘Metals in the service of Man, Alexander and street/pelican 1976: ‘Alumina (aluminium oxide) has a very high melting point of over 2000° C so that it cannot readily be liquefied.However conversion of alumina to aluminium and oxygen, by electrolysis can occur when it is dissolved in some other substance’.
(i) Which solution is used to react with bauxite as a first step in obtaining pure aluminium oxide?
(ii) The aluminium oxide for the electrolytic extraction of aluminum is obtained by heating aluminium hydroxide. Write the equation for this reaction
(iii) Name the element which serves both as the anode and the cathode in the extraction of aluminum.
(iv) Write the equation for the reaction that occurs at the cathode during the extraction of aluminium by electrolysis.
(v) Give the equation for the reaction at the anode when aluminum is purified by electrolysis.
Ans: (i) Sodium hydroxide solution.
(ii) 2Al(OH)3 —> Al2O3 +3H2O
(iii) Graphite
(iv) Reaction at cathode: Al3+ + 3e- —> Al
(v) Reaction at anode: Al – 3e-—> Al3+
Question 1(2008): The following is a sketch of an electrolytic cell used in the extraction of aluminium:
(i) What is the substance of which the electrodes A and B are made?
(ii) At which electrode (A or B) is the aluminum formed?
(iii) What are the two aluminum compounds in the electrolyte C?
(v) why is it necessary for electrode B to be continuously replaced?
Ans: (i) Both electrodes are made of graphite.
(ii) Aluminium metal is produced at electrode A (the cathode).
(iii) The electrolyte C contains alumina (Al₂O₃) and cryolite (Na₃AlF₆).
(iv) The anode (electrode B) is continuously replaced because it reacts with the oxygen produced and gets oxidized, causing it to burn away.
Question 2(2008): Brass is an alloy of:
A. Copper and tin,
B. Copper and Zic
C. Zinc and lead,
D. Lead and tin.
Ans: Brass is an alloy of copper and Zinc.