NCERT Solutions for Class 8 Science Chapter 4
Combustion is a scientific term for burning. It’s a chemical reaction between a substance (fuel) and oxygen that gives off heat. The fuel can be solid, liquid or gas, like wood, candle wax, or propane. Light is also sometimes released during combustion, which creates flames.
Experiments in your textbook show that you need oxygen for combustion to happen. Without enough oxygen, a fire will sputter and die out.
There are different types of combustion. Rapid combustion, like burning natural gas in a stovetop, happens very quickly. In contrast, slow combustion, like rusting, happens gradually over a long time.
Flames themselves have a structure. The different zones within a flame have different temperatures, with the hottest part being at the top.
Finally, the burning of fuels can also produce harmful products like smoke and carbon dioxide, so it’s important to be aware of these.
NCERT Solutions for Class 8 Science Chapter 4
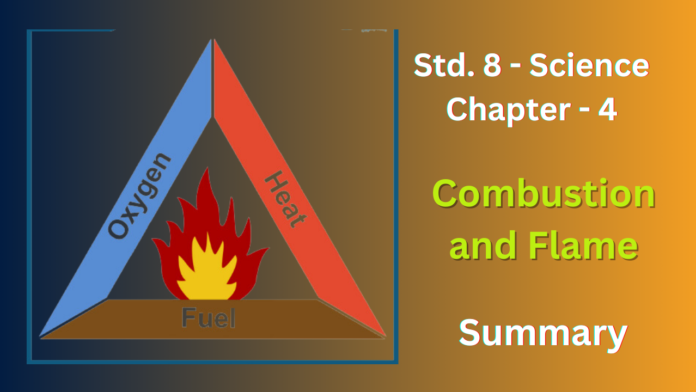
Q1. List conditions under which combustion can take place.
Ans: There are three main conditions needed for combustion to take place:
- Presence of a fuel: This is the combustible material that will burn. It can be a solid, liquid, or gas like wood, gasoline, or natural gas.
- Presence of oxygen (oxidizer): Oxygen is usually obtained from the air and is necessary for the combustion reaction to occur.
- Attainment of ignition temperature: The fuel needs to be heated to a specific temperature, called its ignition temperature, before it will ignite and start burning.
Q2. Fill in the blanks.
(a) Burning of wood and coal causes _____ of air.
(b) A liquid fuel, used in homes is ______
(c) Fuel must be heated to its ______ before it starts burning.
(d) Fire produced by oil cannot be controlled by ______
Ans:
(a) pollution
(b) kerosene
(c) ignition temperature
(d) water.
Q3. Explain how the use of CNG in automobiles has reduced pollution in our cities.
Ans: CNG burns cleaner than gasoline and diesel, reducing harmful pollutants and emissions like smog-causing hydrocarbons and acid rain linked nitrogen oxides. It also skips the lead and soot of traditional fuels. This all adds up to cleaner air in cities.
Q4. Compare LPG and wood as fuels.
Ans: LPG is the cleaner, more convenient option: easy to use, burns hot with minimal smoke. Wood is cheaper but requires storage, pollutes more, and takes more effort to use.
Q5. Give reasons.
(a) Water is not used to control fires involving electrical equipment.
(b) LPG is a better domestic fuel than wood.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not..
Ans:
(a) Water shocks! It conducts electricity in electrical fires.
(b) Cleaner and easier! LPG wins over wood for homes.
(c) Metal steals the heat! Aluminium conducts heat away from paper, keeping it cool
Q6.Make a labelled diagram of a candle flame.
Outer Zone (Blue) – Hottest, Complete Combustion
/\
/ \
/ \
/ \
/ \
Middle Zone (Yellow) – Hot, Incomplete Combustion
/__________\
| |
| Inner Zone | – Dark, Unburnt Fuel
| |
| |
Q7. Name the unit in which the calorific value of a fuel is expressed.
Ans: The calorific value of a fuel is most commonly expressed in kilojoules per kilogram (kJ/kg) within the International System of Units (SI).
Q8. Explain how CO2 is able to control fires.
Ans: CO2 cuts off the fire’s oxygen supply by smothering it with a heavy gas, and cools the fire by rapidly expanding.
Q9. It is difficult to burn a heap of green leaves but dry leaves catch fire easily. Explain.
Ans: Green leaves are full of water, which acts like a fire extinguisher. It absorbs heat that would ignite the leaves (ignition temperature) and reduces the amount of actual fuel (cellulose) available to burn. Dry leaves burn easily because they have less water to hinder these processes.
Q10. Which zone of a flame does a goldsmith use for melting gold and silver and why?
Ans: A goldsmith uses the outermost zone (also called the non-luminous zone) of a flame for melting gold and silver. This zone is the hottest part of the flame because it has the highest temperature due to complete combustion. The high temperature in this zone efficiently melts gold and silver.
Q11. In an experiment, 4.5 kg of fuel was completely burnt. The heat produced was measured to be 180,000 kJ. Calculate the calorific value of the fuel.
Ans: Certainly! To calculate the calorific value of the fuel, we can use the following formula:
Calorific Value (CV) = Heat Produced (HP) / Mass of Fuel (M)
Here’s how we can solve this:
- Given values:
- Heat Produced (HP) = 180,000 kJ
- Mass of Fuel (M) = 4.5 kg
- Calculation:
- CV = HP / M = 180,000 kJ / 4.5 kg
- Result:
- CV = 40,000 kJ/kg (rounded to two significant digits)
Therefore, the calorific value of the fuel is 40,000 kJ/kg. This indicates that for every kilogram of this fuel burnt completely, it releases 40,000 kilojoules of heat energy.
Q12. Can the process of rusting be called combustion? Discuss.
Ans: Rusting and combustion are both oxidation reactions with some heat release, but rusting is much slower, doesn’t need high temperatures or produce light, and can happen with less oxygen. So, rusting is more like a slow burn, not a true combustion
Q13. .Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick in the yellow part of the candle flame. Ramesh kept the beaker in the outermost part of the flame. Whose water will get heated in a shorter time?
Ans: Ramesh’s water will get heated in a shorter time.
The outermost part of the candle flame, which is blue in color, is the hottest zone due to complete combustion. Since the temperature is higher in this zone, it will transfer heat to the beaker faster, heating the water more quickly. The yellow zone, where Abida placed her beaker, is less hot because combustion is incomplete there.
NCERT Solutions for Class 8 Science Chapter 4
FAQ’s
What concepts are covered in Class 8 Science Chapter 4 regarding combustion and flame?
Class 8 Science Chapter 4 delves into the principles of combustion, exploring the process of burning and the characteristics of different types of flames.
How can NCERT Solutions for Class 8 Science Chapter 4 aid in understanding combustion and flame?
NCERT Solutions for Class 8 Science Chapter 4 provide comprehensive explanations and examples to grasp the concepts of combustion and flame effectively.
Are there practical applications discussed in Class 8 Science Chapter 4 related to combustion and flame?
Yes, Class 8 Science Chapter 4 elucidates the practical applications of combustion and flame in various everyday scenarios, such as cooking, heating, and industrial processes.
Can Class 8 Science Chapter 4 explain the safety measures to prevent accidents related to combustion?
Absolutely! Class 8 Science Chapter 4 discusses important safety precautions to prevent accidents associated with combustion, promoting awareness and responsible handling of fire.
How do different types of flames vary in Class 8 Science Chapter 4, focusing on combustion and flame?
Class 8 Science Chapter 4 categorizes flames based on their characteristics, such as luminosity and temperature, providing insights into their properties and applications.