1. Introduction to Organic Chemistry
It is the study of carbon compounds, especially hydrocarbons (compounds of carbon and hydrogen).
Carbon’s key property is catenation – the ability to form long chains and rings, leading to a vast number of compounds.
2. Hydrocarbons: The Fundamentals
They are classified based on the types of carbon-carbon bonds:
Saturated Hydrocarbons (Alkanes): Have only single bonds (-C-C-). General formula: CₙH₂ₙ₊₂. Example: Methane (CH₄), Ethane (C₂H₆).
Unsaturated Hydrocarbons:
Alkenes: Have at least one double bond (-C=C-). General formula: CₙH₂ₙ. Example: Ethene (C₂H₄).
Alkynes: Have at least one triple bond (-C≡C-). General formula: CₙH₂ₙ₋₂. Example: Ethyne (C₂H₂).
3. Functional Groups
A functional group is an atom or group of atoms that determines the chemical properties of an organic compound.
Halogen: -X (Cl, Br, etc.) e.g., Chloromethane (CH₃Cl)
Hydroxyl: -OH (Alcohols) e.g., Ethanol (C₂H₅OH)
Aldehyde: -CHO e.g., Ethanal (CH₃CHO)
Ketone: >C=O e.g., Acetone (CH₃COCH₃)
Carboxylic Acid: -COOH e.g., Acetic Acid (CH₃COOH)
4. Key Reactions
Substitution Reaction: A characteristic of alkanes. One atom is replaced by another. (e.g., Methane with chlorine in sunlight).
Addition Reaction: A characteristic of alkenes and alkynes. Atoms add across the double/triple bond. (e.g., Ethene with hydrogen to form ethane).
Combustion: Burns in oxygen to give CO₂, H₂O, and heat.
Oxidation: Alcohols can be oxidized to aldehydes and then to carboxylic acids using oxidizing agents like K₂Cr₂O₇.
Esterification: Reaction between a carboxylic acid and an alcohol in the presence of concentrated H₂SO₄ to form a sweet-smelling ester.
Saponification: Alkaline hydrolysis of esters (fats) to make soap.
5. Important Homologous Series
A series of compounds where each member differs from the next by a -CH₂ group and has the same general formula and chemical properties.
Alkanes, Alkenes, Alkynes
Alcohols: -OH functional group. e.g., Methanol, Ethanol.
6. Specific Compounds & Their Preparation
Ethanol: Hydration of Ethene or fermentation of sugars.
Ethanoic Acid (Acetic Acid): Oxidation of Ethanol.
Ethene: Dehydration of Ethanol with conc. H₂SO₄.
Ethyne: Action of water on Calcium Carbide.
This chapter forms the basic foundation for understanding the structure, classification, and fundamental reactions of simple organic molecules.
EXERCISE 12(A)
1. Write the IUPAC name of the following:
a)CH₃
|
CH₃ — C — CH₃
|
CH₃
b)CH₃ — CH — CH₂ — CH₃
|
CH₃
c)H — C = C — C — H
|
CH₃
d)H₂C — C — CH₂CH₂CH₃
|
CH₃
e)CH₃ — C ≡ C — CH₂CH₃
f) H — C ≡ C — C — H
|
CH₃
g)CH₃ — CH — CH — CH₂CH₃
| |
Cl CH₃
h)CH₃ — CH — CH₂
|
CH₂CH₂CH₃
i)CH₃ — CH — CH₂CH₃
|
CH₃ — C ≡ C — CH₂CH₂CH₃
k)CH₃ — C — CH₂CH₂CH₂CHO
|
CH₃
l)CH₃ — CH — CH₂CH₂CH₃
|
OH
m)CH₃CHCH₂CH₂COOH
|
CH₃
n) CH₃
|
CH₃–C–CH₂–CH₃
|
Br
o) CH₃
|
CH₃–C–CH₂–CH₂–Br
|
I
p) H
|
H–C–C≡C–H
|
I
(q) H H H H H
| | | | |
H – C – C – C – C – C – H
| | | | |
H H H H H
( r) H H H
| | |
H – C – C – C – H
| | |
H H H
|
C – H
| |
H H
(s) H O
| |
H – C – C
| |
H OH
(t) H H
| |
H – C – C – H
| |
Cl Cl
Ans: a) 2,2-dimethylpropane
b) 2-methylbutane
c) but-2-yne
d) 2-methylpentane
e) hex-2-yne
f) but-1-yne
g) 2-chloro-3-methylpentane
h) 3-methylpentane
i) 3-methylhex-1-yne
k) 5,5-dimethylhexanal
l) pentan-2-ol
m) 4-methylpentanoic acid
n) 2-bromo-2-methylbutane
o) 1-bromo-3-iodo-3-methylbutane
p) 1-iodoethyne
q) pentane
r) 2-methylpropane
s) methanoic acid
t) 1,2-dichloroethane
2. Write the structures of the following compounds:
(a) Prop-1-ene
(b) 2,3-dimethylbutane
(c) 2-methylpropane
(d) 3-hexene
(e) Prop-1-yne
(f) 2-methylprop-1-ene
(g) Alcohol with molecular formula C4H10O
Ans:(a) CH₃-CH=CH₂
H H
| |
H – C – C = C – H
| |
H H
(b) CH₃-CH(CH₃)-CH(CH₃)-CH₃
CH₃ CH₃
| |
CH₃ – C – C – CH₃
| |
H H
(c) (CH₃)₃CH
CH₃
|
CH₃ – C – CH₃
|
H
(d) CH₃-CH₂-CH=CH-CH₂-CH₃
H H H H
| | | |
C = C – C – C – C – C
| | | | |
H H H H H
(e)CH≡C-CH₃
H – C ≡ C – CH₃
(f)(CH₃)₂C=CH₂
CH₃
|
H – C – C = CH₂
|
H
(g) CH₃-CH₂-CH₂-CH₂OH
H H H H
| | | |
C – C – C – C – O – H
| | | |
H H H H
3. Choose the correct answer:
(a) C5H11is an
(i) alkane
(ii) alkene
(iii) alkyne
(iv) alkyl group
(b) A hydrocarbon of the general formula CnH2n+2
(i) C7H12
(ii) C7H16
(iii) C8H20
(iv) C8H14
(c) A hydrocarbon with molecular mass 72 is
(i) an alkane (ii) an alkene (iii) an alkyne
(d) The total number of different carbon chains that four carbon atoms form in alkane is
(i) 5 (ii) 4 (iii) 3 (iv) 2
(e) CH3−CH2−OH and CH3−O−CH3
(i) position isomers (ii) chain isomers (iii) homologous (iv) functional-group isomers
(f) The IUPAC name of the compound
CH3−CH2−CH2−CH(CH3)−CH2−CH3 is
(i) 3-trimethylhexane (ii) 3-methylhexane (iii) 4-methylhexane
Ans: (a) (iv) alkyl group
(b) (ii) C₇H₁₆
(c) (ii) an alkene
(d) (iv) 2
(e) (iv) functional-group isomers
(f) (ii) 3-methylhexane
4. Fill in the blanks.
(a) Propane and ethane are …… . (homologous, isomers)
(b) A saturated hydrocarbon does not participate in a/an …… reaction (substitution, addition)
(c) Succeeding members of a homologous series differ by …… . (CH, CH2, CH3)
(d) As the molecular masses of hydrocarbons increase, their boiling points …… and melting points …… (increase, decrease)
(e) C25H52 and C50H102 belong to ….. homologous series (the same, different)
(f) CO is an …… compound. (organic, inorganic)
(g) The chemical properties of an organic compound are largely decided by the …… and the physical properties of an organic compound are largely decided by the …… (functional group, number of carbon atoms)
(h) CHO is the functional group of an …… . (alcohol, aldehyde)
(i) The root in the IUPAC name of an organic compound depends upon the number of carbon atoms in …… . (any chain, principal chain)
(j) But-1-ene and but-2-ene are examples of …… isomerism. (chain, position, functional)
Ans:
(a) Propane and ethane are homologous.
(b) A saturated hydrocarbon does not participate in an addition reaction.
(c) Succeeding members of a homologous series differ by CH₂.
(d) As the molecular masses of hydrocarbons increase, their boiling points increase and melting points increase.
(e) C₂₅H₅₂ and C₅₀H₁₀₂ belong to the same homologous series.
(f) CO is an inorganic compound.
(g) The chemical properties of an organic compound are largely decided by the functional group and the physical properties of an organic compound are largely decided by the number of carbon atoms.
(h) CHO is the functional group of an aldehyde.
(i) The root in the IUPAC name of an organic compound depends upon the number of carbon atoms in the principal chain.
(j) But-1-ene and but-2-ene are examples of position isomerism.
5. Draw structural formula for each of the following compounds:
(a) isomer of n-butane
(b) vinegar
(c) 2-propanol
(d) ethanol
(e) acetone
(f) diethyl ether
What is used to describe these compounds taken together?
Ans:
(a) isomer of n-butane
CH₃
|
CH₃-C-CH₃
|
H
(b) vinegar
O
||
CH₃-C-OH
(c) 2-propanol
CH₃
|
CH₃-C-OH
|
H
(d) ethanol
CH₃-CH₂-OH
(e) acetone
O
||
CH₃-C-CH₃
(f) diethyl ether
CH₃-CH₂-O-CH₂-CH₃
These compounds are collectively described as organic compounds. More specifically, they are a group of oxygen-containing organic compounds or a mixture of different functional groups like alkanes, alcohols, carboxylic acids, and ethers.
6.(a) What is the special feature of the structure of:
(i) C2H4
(ii) C2H2
(b) What type of reaction is common to both these compounds? Why methane does not undergo this type of reaction.
Ans: (a) Special feature of structure:
(i) C₂H₄ (Ethene): It contains a carbon-carbon double bond (C=C), making it planar with bond angles of approximately 120°.
(ii) C₂H₂ (Ethyne): It contains a carbon-carbon triple bond (C≡C), giving it a linear structure with bond angles of 180°.
(b) Common reaction:
Both compounds undergo addition reactions due to the presence of multiple bonds (double or triple), which can break to form single bonds with added atoms.Methane (CH₄) does not undergo addition reactions because it has only single bonds (saturated hydrocarbon) and lacks π-electrons necessary for addition. Instead, methane undergoes substitution reactions.
7. Give the names and structural formula of:
(a) saturated hydrocarbon
(b) unsaturated hydrocarbon
Which type of reaction will they undergo?
Ans:
(a) Saturated hydrocarbon
Name: Ethane
H H
| |
H – C – C – H
| |
H H
Reaction type: Substitution reaction (e.g., with chlorine in sunlight)
(b) Unsaturated hydrocarbon
Name: Ethene (Ethylene)
Structural formula:
H H
| |
C = C
| |
H H
Reaction type: Addition reaction (e.g., with hydrogen, halogens, or water)
8. Choosing only words from the following list, write down appropriate words to fill in the blanks from (a) to (e) given below.
List: Addition, carbohydrates, CnH2n−2, CnH2n, CnH2n+2, electrochemical, homologous, hydrocarbon, saturated, substitution, unsaturated.
The alkanes form an (a) …… series with the general formula (b) …… . The alkanes are (c) …… (d) …… which generally undergo (e) …… reactions.
Ans: (a) homologous
(b) CnH2n+2
(c) saturated
(d) hydrocarbon
(e) substitution
9. Draw the structural formula of a compound with two carbon atoms in each of the following cases.
(a) An alkane with a carbon to carbon single bond
(b) An alcohol containing two carbon atoms
(c) An unsaturated hydrocarbon with a carbon to carbon triple bond
Ans:
(a) An alkane with a carbon to carbon single bond
H H
| |
H-C-C-H
| |
H H
(b) An alcohol containing two carbon atoms
H H
| |
H-C-C-O-H
| |
H H
(c) An unsaturated hydrocarbon with a carbon to carbon triple bond
H-C≡C-H
10. Ethane, Ethene, Ethanoic acid, Ethyne, Ethanol From the above, name:
(a) The compound with -OH as the part of its structure
(b) The compound with -COOH as the part of its structure
(c) Homologue of Homologous series with general formula CnH2n
Ans: (a) Ethanol – contains the -OH (hydroxyl) group.
(b) Ethanoic acid – contains the -COOH (carboxyl) group.
(c) Ethene – belongs to the alkene series with general formula CnH2n
11. Give the correct IUPAC name and the functional group for each of the compounds whose structural formulae are given below:
(a) H H O
| | |
H – C – C – C – H
| |
H H
(b)
H H H
| | |
H – C – C – C – OH
| | |
H H H
Ans:
(a)IUPAC Name: Propanal
Functional Group: Aldehyde (–CHO)
(b)IUPAC Name: Propan-1-ol
Functional Group: Alcohol (–OH)
EXERCISE 12B
1. State the sources of Alkanes.
Ans: Alkanes are primarily obtained from:
Natural gas – Mainly methane with smaller amounts of ethane, propane, and butane.
Petroleum (crude oil) – A major source through fractional distillation.
Coal gas – Obtained during the destructive distillation of coal.
2. Methane is a green house gas. Comment.
Ans: Methane (CH₄) is considered a greenhouse gas because it traps heat in the Earth’s atmosphere, contributing to global warming.
Although it is present in much lower concentrations than carbon dioxide (CO₂), methane is over 25 times more effective at trapping heat over a 100-year period.
Major sources of methane include agricultural activities (like livestock farming and rice cultivation), the decay of organic waste in landfills, and the production and transport of fossil fuels (like natural gas and coal mining).
Therefore, controlling methane emissions is crucial for mitigating climate change.
3. Give the general formula of alkanes.
Ans: The general formula for a group of organic compounds is like a recipe that tells you how many atoms of each element are in any member of that family. For the alkanes, which are a major group of saturated hydrocarbons, this general formula is:
CₙH₂ₙ₊₂
Let’s break down what this means:
Cₙ: This part signifies the number of carbon (C) atoms in the molecule. The ‘n’ is a variable that can be any whole number, starting from 1 (for methane). So, if n=1, you have 1 carbon atom. If n=5, you have 5 carbon atoms.
H₂ₙ₊₂: This part tells you the number of hydrogen (H) atoms. You calculate it by multiplying the number of carbon atoms (n) by 2 and then adding 2 more hydrogen atoms.
Why this specific formula?
The reason for the “2n+2” pattern lies in the molecular structure of alkanes. They are called “saturated” hydrocarbons because every carbon atom forms the maximum number of single bonds (four). In a carbon chain:
The two end carbon atoms each bond with three hydrogen atoms.Every carbon atom in the middle of the chain bonds with two hydrogen atoms.This specific bonding pattern consistently results in the number of hydrogen atoms always being two more than twice the number of carbon atoms.
Examples to Illustrate:
If n = 1 (Methane):
Carbon atoms = 1
Hydrogen atoms = (2 × 1) + 2 = 4
Molecular Formula: CH₄
If n = 3 (Propane):
Carbon atoms = 3
Hydrogen atoms = (2 × 3) + 2 = 8
Molecular Formula: C₃H₈
If n = 8 (Octane):
Carbon atoms = 8
Hydrogen atoms = (2 × 8) + 2 = 18
Molecular Formula: C₈H₁₈
4. Draw the structures of isomers of :
(a) butane
(b) pentane
Write the IUPAC and common names of these isomers.
Ans: (a) butane
Butane (C₄H₁₀) has two structural isomers.
1) n-Butane (or Butane)
IUPAC Name: Butane
Common Name: n-Butane
Structure:
H H H H
| | | |
H–C – C – C – C – H
| | | |
H H H H
CH₃–CH₂–CH₂–CH₃
2)2-Methylpropane
IUPAC Name: 2-Methylpropane
Common Name: Isobutane
Structure:
H
|
H – C – H
|
H–C – H
/ \
H–C C – H
| |
H H
CH₃–CH(CH₃)–CH₃
(or simplified as (CH₃)₃CH)
(b) pentane
Pentane (C₅H₁₂) has three structural isomers.
1)n-Pentane (or Pentane)
IUPAC Name: Pentane
Common Name: n-Pentane
Structure:
H H H H H
| | | | |
H–C – C – C – C – C – H
| | | | |
H H H H H
CH₃–CH₂–CH₂–CH₂–CH₃
2)2-Methylbutane
IUPAC Name: 2-Methylbutane
Common Name: Isopentane
Structure:
H
|
H – C – H
|
H – C – H
/ \
H–C C – C – H
| | | |
H H H H
CH₃–CH(CH₃)–CH₂–CH₃
3)2,2-Dimethylpropane
IUPAC Name: 2,2-Dimethylpropane
Common Name: Neopentane
Structure:
H
|
H – C – H
|
H – C – H
/ | \
H–C C C – H
| | |
H H H
C(CH₃)₄
5. Write the :
(a) molecular formula
(b) electron dot formula and
(c) structural formula
of methane and ethane.
Ans:
(a) Molecular formula:
Methane: CH₄
Ethane: C₂H₆
(b) Electron dot formula:
1)Methane:
H
H : C : H
H
2)Ethane.
H H
H : C : C : H
H H
(c) 1) structural formula of methane
H
|
H — C — H
|
H
2)structural formula of ethane.
H H
| |
C — C
| |
H H
6. How is :
(a) methane and
(b) ethane
prepared in the laboratory ?
Ans:
(a) Laboratory preparation of methane:
Methane is prepared by heating a mixture of sodium acetate and soda lime.
Chemical equation:
CH3COONa+NaOH→ CH4+Na2CO3
(b) Laboratory preparation of ethane:
Ethane is prepared by heating a mixture of sodium propionate with soda lime.
Chemical equation:
CH3CH2COONa+NaOH→C2H6+Na2CO3
7. How are methane and ethane prepared from methyl iodide and ethyl bromide ?
Ans: Methane from methyl iodide:
Methyl iodide reacts with zinc and hydrochloric acid:
CH3I+2H (from Zn + HCl)→CH4+HI
This is a reduction reaction.
Ethane from ethyl bromide:
Ethyl bromide reacts with sodium in dry ether:
2CH3CH2Br+2Na→CH3CH2–CH2CH3+2NaBr
This is the Wurtz reaction, giving n-butane as the major product.
To specifically prepare ethane, methyl bromide can be used instead:
2CH3Br+2Na→CH3–CH3+2NaBr
8. What is a substitution reaction ?
Give the reaction of chlorine with ethane and name the product formed.
Ans: A substitution reaction is a chemical reaction in which one atom or a group of atoms in a molecule is replaced by another atom or group of atoms.
Reaction of chlorine with ethane:
In the presence of sunlight (UV light), chlorine replaces a hydrogen atom in ethane.
C2H6+Cl2→UV LightC2H5Cl+HCl
Product formed: Chloroethane
9. Name the compounds formed when methane burns in :
(a) sufficient air
(b) insufficient air
Give a balanced equation.
Ans: (a) Complete Combustion
When methane burns completely in sufficient air, it reacts with oxygen to form carbon dioxide and water vapour.
Balanced Equation:
CH₄ + 2O₂ → CO₂ + 2H₂O
(b) Incomplete Combustion
When methane burns in a limited supply of air, it forms carbon monoxide and water vapour instead.
Balanced Equation:
2CH₄ + 3O₂ → 2CO + 4H₂O
10. Write the names and the formula of the products formed when :
(a) methane
(b) ethane
reacts with : (i) chlorine (ii) bromine
Write the chemical equations.
Ans: (a) Methane (CH₄)
(i) Reaction with Chlorine
In the presence of UV light, methane reacts with chlorine in a substitution reaction.
Equation:
CH₄ + Cl₂ → CH₃Cl + HCl
Products:Chloromethane (CH₃Cl),Hydrogen chloride (HCl)
(ii) Reaction with Bromine
Similarly, methane undergoes a substitution reaction with bromine when exposed to UV light.
Equation:
CH₄ + Br₂ → CH₃Br + HBr
Products:Bromomethane (CH₃Br),Hydrogen bromide (HBr)
(b) Ethane (C₂H₆)
(i) Reaction with Chlorine
Ethane reacts with chlorine under UV light, substituting one hydrogen atom.
Equation:
C₂H₆ + Cl₂ → C₂H₅Cl + HCl
Products:
Chloroethane (C₂H₅Cl),Hydrogen chloride (HCl)
(ii) Reaction with Bromine
In the presence of UV light, ethane substitutes a hydrogen atom with a bromine atom.
Equation:
C₂H₆ + Br₂ → C₂H₅Br + HBr
Products:Bromoethane (C₂H₅Br),Hydrogen bromide (HBr)
11. Name the compound prepared from :
(a) sodium propionate
(b) methyl iodide and
(c) ethyl bromide
Write a balanced equation for the same.
Ans: (a) Sodium propionate
On dry distillation with soda lime, it gives ethane.
CH3CH2COONa+NaOH→CH3+Na2CO3
(b) Methyl iodide
When treated with sodium in dry ether (Wurtz reaction), it forms ethane.
2CH3I+2Na→CH3–CH3+2NaI
(c) Ethyl bromide
On heating with alcoholic KOH, it undergoes dehydrohalogenation to form ethene.
CH3CH2Br+KOH (alc.)→CH2=CH2+KBr+H2O
12. What is pyrolysis or cracking ? Explain with example.
Ans: Pyrolysis or Cracking
Pyrolysis, also called cracking, is a chemical process where large hydrocarbon molecules are broken down into smaller, more useful molecules by heating them to high temperatures in the absence of air (or with limited air). This process is commonly used in the petroleum industry to convert heavy oil fractions into lighter, more valuable products like petrol.
Example:
When kerosene (a heavy hydrocarbon) is heated strongly in the absence of air, it breaks down into smaller hydrocarbons such as petrol and ethene.
C10H22→C8H18+C2H4
(Dodecane → Octane + Ethene)
13. Convert :
(a) Methane into chloroform
(b) Sodium acetate into methane
(c) Methyl iodide into ethane
(d) Aluminium carbide into methane
Ans: (a) Methane into chloroform
When methane reacts with chlorine in the presence of light or heat, chloroform is formed:
CH₄ + 3Cl₂ → CHCl₃ + 3HCl
(b) Sodium acetate into methane
Heating sodium acetate with soda lime (NaOH + CaO) gives methane:
CH₃COONa + NaOH → CH₄ + Na₂CO₃
(c) Methyl iodide into ethane
Methyl iodide reacts with sodium metal in dry ether to form ethane (Wurtz reaction):
2CH₃I + 2Na → C₂H₆ + 2NaI
(d) Aluminium carbide into methane
Aluminium carbide on hydrolysis produces methane:
Al₄C₃ + 12H₂O → 3CH₄ + 4Al(OH)₃
14. Give three uses of :
(a) methane
(b) ethane
Ans: (a) Uses of Methane:
Used as a fuel for cooking and heating in homes and industries.
Helps generate electricity in power stations.
Acts as a raw material to produce chemicals like hydrogen and methanol.
(b) Uses of Ethane:
Mainly used to make ethylene, which is important for producing plastics.
Sometimes used in refrigeration systems.
Used as a fuel in certain industrial processes.
15. Under what conditions does ethane get converted to :
(a) ethyl alcohol
(b) acetaldehyde
(c) acetic acid
Ans: (a) Ethyl alcohol:
Ethane is first converted to ethene (by heating with steam and a catalyst), then ethene undergoes hydration with steam in the presence of an acid catalyst to form ethyl alcohol.
(b) Acetaldehyde:
Ethane is oxidized in the presence of a suitable catalyst (e.g., manganese acetate) at high temperature and pressure to form acetaldehyde.
(c) Acetic acid:
Ethane can be catalytically oxidized with oxygen in the presence of a cobalt or manganese catalyst at high temperature and pressure to form acetic acid directly.
Alternatively, acetaldehyde formed from ethane can be further oxidized to acetic acid.
16. Give the inter-relationship of methane, methyl alcohol, formaldehyde and formic acid with conditions.
Ans: Here’s the inter-relationship of methane, methyl alcohol, formaldehyde, and formic acid with conditions:
Methane (CH₄) → Methyl alcohol (CH₃OH)
Condition: Oxidation with steam and catalyst at high temperature and pressure.
Methyl alcohol (CH₃OH) → Formaldehyde (HCHO)
Condition: Catalytic oxidation (with Cu or Ag catalyst at 573 K).
Formaldehyde (HCHO) → Formic acid (HCOOH)
Condition: Oxidation with acidified potassium permanganate (KMnO₄) or potassium dichromate (K₂Cr₂O₇).
These steps show a progressive oxidation sequence from methane to formic acid.
EXERCISE 12C
1. Write :
(a) molecular formula
(b) electron dot formula and
(c) structural formula
of ethene (ethylene).
Ans: (a) Molecular formula:C₂H₄
(b) Electron dot formula:
H H
• •
H : C : : C : H
• •
H H
(c) Structural formula:
H–C=C–H
| |
H H
2. The molecules of alkene family are represented by a general formula CnH2n
Answer the following:
(a) What do n and 2n signify?
(b) What is the name of alkene when n=4?
(c) What is the molecular formula of alkene when n=4?
(d) What is the molecular formula of the alkene if there are ten H atoms in it?
(e) What is the structural formula of the third member of the alkene family?
(f) Write the molecular formula of lower and higher homologous of an alkene which contains four carbon atoms.
Ans: (a)In the general formula for alkenes, n represents the number of carbon atoms, and 2n represents the number of hydrogen atoms.
(b)When n = 4, the alkene is called Butene.
(c)For n = 4, the molecular formula is C₄H₈.
(d)If the alkene has 10 hydrogen atoms, then:
2n = 10 → n = 5
Molecular formula: C₅H₁₀
(e)The third member of the alkene family is Propene (n = 3).
Its structural formula is:
CH₂ = CH – CH₃
(f)Alkene with 4 carbon atoms: C₄H₈
Its lower homologue (n = 3): C₃H₆
Its higher homologue (n = 5): C₅H₁₀
3. Draw the structures of butene and write IUPAC names.
Ans: 1. But-1-ene
Structure: CH₂=CH–CH₂–CH₃
IUPAC Name: But-1-ene
2. But-2-ene
This isomer has two forms (cis and trans) due to restricted rotation around the double bond.
(a) cis-But-2-ene
Structure:
H₃C H
\ /
C = C
/ \
H CH₃
IUPAC Name: (Z)-But-2-ene
(b) trans-But-2-ene
Structure:
H₃C CH₃
\ /
C = C
/ \
H H
IUPAC Name: (E)-But-2-ene
3. 2-Methylprop-1-ene
Structure: CH₂=C(CH₃)₂
IUPAC Name: 2-Methylprop-1-ene
4. Give a balanced equation for the lab. preparation of ethylene. How is the gas collected?
Ans: Balanced Equation:
C2H5OH→CH2=CH2+H2O
Ethanol undergoes dehydration using concentrated sulphuric acid at 170°C to form ethene (ethylene) and water.
Collection of Gas:
The gas is collected by the downward displacement of water because ethene is only sparingly soluble in water.
5. How is ethene prepared by :
(a) dehydrohalogenation reaction?
(b) dehydration reaction?
Give equations and name the products formed.
Ans:
(a) Dehydrohalogenation Reaction
Ethene is prepared by heating a haloalkane (e.g., bromoethane) with a concentrated alcoholic solution of potassium hydroxide (KOH).A hydrogen atom and a halogen atom are removed from adjacent carbon atoms, forming ethene.
Equation:
CH3−CH2Br+KOH (alc.)→CH2=CH2+KBr+H2O
Products: Ethene, potassium bromide, and water.
(b) Dehydration Reaction
Ethene is prepared by heating ethanol with excess concentrated sulphuric acid at about 170°C.
Equation:
CH3−CH2OH→CH2=CH2+H2O
Products: Ethene and water.
6. (a) Ethylene when reacts with halogens (chlorine and bromine) form saturated products. Name them and write balanced equations.
(b) Give the conditions and the main product formed by hydrogenation of ethylene.
Ans: (a)Ethylene reacts with chlorine to form 1,2-dichloroethane, and with bromine to form 1,2-dibromoethane.
Balanced equations:
C₂H₄ + Cl₂ → C₂H₄Cl₂
C₂H₄ + Br₂ → C₂H₄Br₂
(b)Hydrogenation of ethylene occurs in the presence of a nickel catalyst at around 200–300 °C.
The main product formed is ethane (C₂H₆).
Reaction:
C₂H₄ + H₂ → C₂H₆
7. How is ethanol converted into ethene using
(a) solid dehydrating agent
(b) hot conc. H2SO4? Give only balanced equations.
Ans: (a) Using solid dehydrating agent (Al₂O₃):
CH3CH2OH→ΔAl2O3CH2=CH2+H2O
(b) Using hot concentrated H₂SO₄:
CH3CH2OH→CH2=CH2+H2O
8. Write the following properties of ethene :
(a) Physical state
(b) Odour
(c) Density as compared to air
(d) Solubility
Ans: (a) Physical state – It is a gas under normal room conditions.
(b) Odour – It has a faint, sweetish smell.
(c) Density compared to air – It is slightly lighter than air.
(d) Solubility – It dissolves only very slightly in water, but mixes well with organic solvents.
9. How would you convert :
(a) ethene into 1,2-dibromoethane?
(b) ethene into ethyl bromide?
Ans: (a) Ethene → 1,2-dibromoethane
Pass ethene through bromine water.
An addition reaction occurs, breaking the double bond.
Colour changes from orange to colourless.
Reaction: C₂H₄ + Br₂ → C₂H₄Br₂
(b) Ethene → Ethyl bromide
React ethene gas with hydrogen bromide (HBr).
An addition reaction occurs, adding H and Br across the double bond.
Reaction: C₂H₄ + HBr → C₂H₅Br
10. Give balanced equations when :
(a) ethene is burnt in excess of oxygen.
(b) ethene reacts with chlorine.
(c) ethene combines with hydrogen chloride.
(d) a mixture of ethene and hydrogen is passed over nickel at 200°C.
Ans: (a)
C₂H₄ + 3O₂ → 2CO₂ + 2H₂O
(b)
C₂H₄ + Cl₂ → C₂H₄Cl₂
(c)
C₂H₄ + HCl → C₂H₅Cl
(d)
C₂H₄ + H₂ → C₂H₆
(Nickel, 200°C)
11. Give the formula and names of A, B, C and D in the following equations :
(a) CH4→CH2A→CH2B→CH2C→CH2D
(b) C2H2→H2A→H2B→Br2C→Br2D
(c) C2H4+Cl2→A
(d) C2H4+B→200°CC2H6
Ans: (a) CH₄ → CH₂A → CH₂B → CH₂C → CH₂D
This series represents the chlorination of methane in the presence of UV light.
A = Cl₂ (Chlorine)
B = Cl₂ (Chlorine)
C = Cl₂ (Chlorine)
D = Cl₂ (Chlorine)
Product Names:
CH₃Cl: Chloromethane (Methyl chloride)
CH₂Cl₂: Dichloromethane (Methylene chloride)
CHCl₃: Trichloromethane (Chloroform)
CCl₄: Tetrachloromethane (Carbon tetrachloride)
(b) C₂H₂ → H₂A → H₂B → Br₂C → Br₂D
This series represents the addition reactions of acetylene.
A = H₂ (Hydrogen)
B = H₂ (Hydrogen)
C = Br₂ (Bromine)
D = Br₂ (Bromine)
Product Names:
C₂H₄: Ethene (Ethylene)
C₂H₆: Ethane
C₂H₄Br₂: 1,2-Dibromoethane
C₂H₄Br₄: 1,1,2,2-Tetrabromoethane
(c) C₂H₄ + Cl₂ → A
This is the addition of chlorine to ethene.
A = C₂H₄Cl₂
Name: 1,2-Dichloroethane (Ethylene dichloride)
(d) C₂H₄ + B → C₂H₆
This is the catalytic hydrogenation of ethene to ethane.
B = H₂ (Hydrogen)
Conditions: Nickel (Ni) catalyst at 200 °C.
12. Write the name and formula of the product formed in each case below :
(a) C2H4+Cl2→………………
(b) C2H5Br+KOH (alc.) →………………
(c) H2C=CH2→alk.KMnO4 ………………
(d) H2C=CH2+HBr→………………
(e) H2C=CH2+O3→………………
Ans: (a) 1,2-Dichloroethane — C₂H₄Cl₂
(b) Ethene — C₂H₄
(c) Ethylene glycol — C₂H₆O₂
(d) Bromoethane — C₂H₅Br
(e) Formaldehyde — HCHO
13. What do you observe when ethylene is passed through alkaline KMnO4 solution?
Ans: When ethylene is passed through alkaline KMnO₄ solution (Baeyer’s test), the purple colour of the solution disappears and a brown precipitate of manganese dioxide (MnO₂) is formed.
14. Name three compounds formed by ethylene and give the use of these compounds.
Ans: Polyethylene
If you look around any room, you are almost certainly looking at polyethylene. It is the workhorse of the plastic world, the most common plastic you will encounter in your daily life. Its popularity comes from its versatility, durability, and low cost.You interact with it in its various forms constantly. The lightweight, flexible plastic bag you carry your groceries in is polyethylene. The squeezable bottle containing your shampoo or laundry detergent is made from it. It’s the sturdy material used for milk jugs, food storage containers, and even the toys children play with. In its tougher forms, it can be found as plastic lumber for decks or the cutting boards in your kitchen. Essentially, polyethylene is the invisible yet essential material that packages, protects, and contains a huge portion of the modern world’s goods.
Ethylene Glycol
Ethylene glycol is best known for its role as a lifesaver for car engines, especially in colder climates. This thick, sweet-tasting liquid has a remarkable property: it dramatically lowers the freezing point of water.This is why it is the primary ingredient in automobile antifreeze and engine coolant. By mixing it with water and circulating it through the engine’s cooling system, it prevents the radiator and engine block from freezing solid and cracking in the winter. Conversely, it also raises the boiling point of the coolant, helping to prevent the engine from overheating in the summer. It’s crucial to handle ethylene glycol with care, as its sweet taste can be dangerously attractive to pets and children, and it is highly toxic if ingested.
Ethylene Oxide
Ethylene oxide operates in a realm that is critical for public health but largely unseen by the general public. It is a potent, gaseous sterilizing agent. Its primary superpower is its ability to penetrate materials and destroy the DNA of microorganisms, including bacteria, viruses, and fungal spores.This makes it indispensable for sterilizing medical and surgical equipment that cannot withstand the high heat of steam sterilization, such as plastic sutures, scalpels, and complex devices like endoscopes. Furthermore, ethylene oxide is a key building block in the chemical industry. A significant portion of its production is used to manufacture ethylene glycol (the antifreeze mentioned above) and is also a key ingredient in the production of many detergents and surfactants that we use for cleaning.
EXERCISE 12D
1. What are the sources for alkynes? Give the general formula of alkynes.
Ans: The main industrial source for acetylene (ethyne) is calcium carbide (CaC₂). This human-made compound is produced by heating coke and lime in a furnace. When calcium carbide is mixed with water, it produces acetylene gas.
Some complex alkynes are found naturally in certain plants, fungi, and marine sponges. However, these natural sources are not significant for large-scale use.
General Formula of Alkynes
The general formula for a straight-chain alkyne is CₙH₂ₙ₋₂.
This is because a carbon-carbon triple bond uses more bonding sites than a single or double bond, resulting in four fewer hydrogen atoms compared to an alkane with the same number of carbon atoms. This makes alkynes highly unsaturated and reactive.
2. Give an example of isomers shown by triple bond hydrocarbon (alkynes) and write its IUPAC name.
Ans: Examples of alkyne isomers:
Compound 1: CH₃−CH₂−C≡C−CH₃
IUPAC Name: Pent-2-yne
Compound 2: CH₃−C≡C−CH₂−CH₃
IUPAC Name: Pent-3-yne
These two structures are positional isomers. Both share the molecular formula C₅H₈ but differ in the location of the triple bond along the carbon chain.
3. How is acetylene prepared in the laboratory?
(a) Draw a diagram.
(b) Give an equation.
(c) How is pure dry gas collected?
Ans: (a) Diagram:
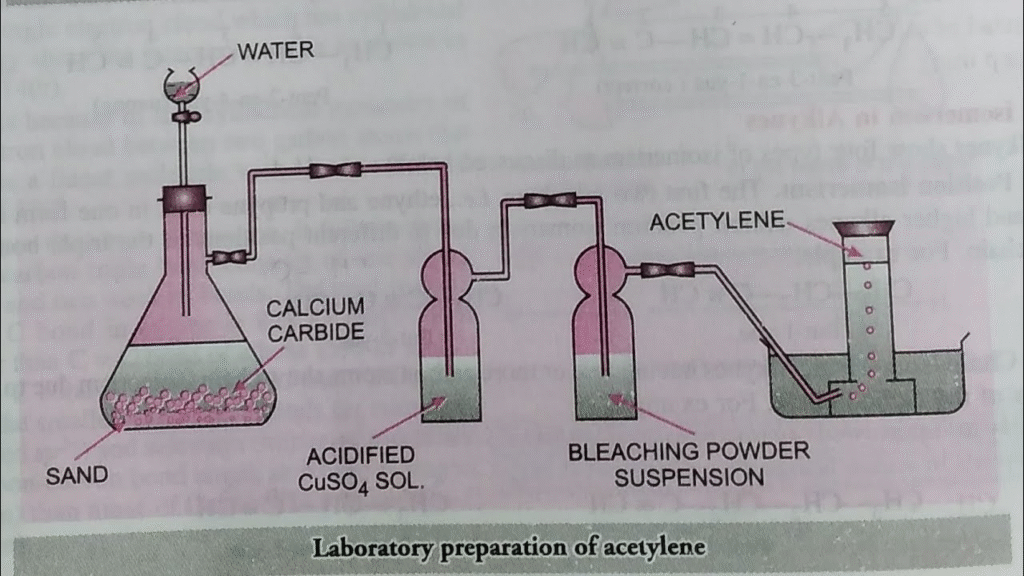
(b) Equation:
CaC₂(s) + 2H₂O(l) → C₂H₂(g) + Ca(OH)₂(aq)
(c) Collection of Pure Dry Gas:
Acetylene gas is collected over water.
It is not dried by passing through concentrated sulfuric acid as it reacts with it. For drying, it can be passed through a drying agent like quicklime (CaO) or anhydrous copper sulfate before collection.
4. Give the method of preparation of ethyne from 1,2-dibromoethene.
Ans: Preparation of Ethyne from 1,2-Dibromoethene
Ethyne can be prepared from 1,2-dibromoethene by dehalogenation. The method involves the removal of bromine atoms using zinc dust in an alcoholic medium.
Reaction:
BrCH=CHBr+Zn→AlcoholHC≡CH+ZnBr2
Procedure:
1,2-Dibromoethene is heated with zinc dust in alcohol (e.g., ethanol). The zinc reacts, removing the bromine atoms and forming a triple bond between the carbon atoms, producing ethyne gas and zinc bromide.
5. Name the hydrocarbon which :
(a) is a tetrahedral molecule
(b) is a planar molecule
(c) is a linear molecule
(d) forms a red precipitate with ammoniacal solution of copper(I) chloride
(e) is known as paraffin
(f) is known as olefin
(g) is a compound which will give acetylene (ethyne) gas when treated with water
Ans: (a) Methane (CH₄) – It has a tetrahedral shape.
(b) Ethene (C₂H₄) – It is planar due to sp² hybridization.
(c) Ethyne (C₂H₂) – It is linear due to sp hybridization.
(d) Ethyne (C₂H₂) – Forms red copper acetylide with ammoniacal Cu₂Cl₂.
(e) Alkane – General name “paraffin” for alkanes.
(f) Alkene – General name “olefin” for alkenes.
(g) Calcium carbide (CaC₂) – On reaction with water gives acetylene.
6. Classify the following compounds as alkanes, alkenes and alkynes:
C3H4,C3H8,C5H12,C3H6
Ans: C₃H₄ → Alkyne
C₃H₈ → Alkane
C₅H₁₂ → Alkane
C₃H₆ → Alkene
7. Give a chemical test to distinguish between:
(a) saturated and unsaturated compounds
(b) ethane and ethene
(c) ethene (ethylene) and ethyne (acetylene)
Ans: (a) Saturated and Unsaturated Compounds
Test: Add bromine water (reddish-brown) and shake.
Saturated Compound: No colour change (remains reddish-brown).
Unsaturated Compound: Colour discharges (becomes colourless).
(b) Ethane and Ethene
Test: Pass the gas through bromine water.
Ethane (saturated): No change; bromine water stays reddish-brown.
Ethene (unsaturated): Decolorizes bromine water; it turns colourless.
(c) Ethene and Ethyne
Test: Use ammoniacal silver nitrate solution (Tollens’ reagent).
Ethene: No reaction or no visible change.
Ethyne: Forms a white precipitate of silver acetylide.
8. Name the products formed and write an equation when ethyne is added to the following in an inert solvent:
(a) chlorine
(b) bromine
(c) iodine
(d) hydrogen
(e) excess of hydrochloric acid
Ans: (a) With Chlorine:
Ethyne reacts with chlorine to form 1,2-dichloroethene.
Equation:
HC≡CH + Cl₂ → ClCH=CHCl
(1,2-dichloroethene)
(b) With Bromine:
Ethyne reacts with bromine to form 1,2-dibromoethene.
Equation:
HC≡CH + Br₂ → BrCH=CHBr
(1,2-dibromoethene)
(c) With Iodine:
Ethyne reacts slowly with iodine to form 1,2-diiodoethene.
Equation:
HC≡CH + I₂ → ICH=CHI
(1,2-diiodoethene)
(d) With Hydrogen:
In the presence of a catalyst like Ni, Pt, or Pd, ethyne adds hydrogen to first form ethene and then ethane.
Equation (to form Ethene):
HC≡CH + H₂ → H₂C=CH₂
(e) With excess Hydrochloric Acid:
Ethyne adds two molecules of HCl to form 1,1-dichloroethane.
Equation:
HC≡CH + 2HCl → CH₃–CHCl₂
(1,1-dichloroethane)
EXERCISE 12E
1. (a) What are alcohols? State their sources.
(b) Give general formulae of monohydric alcohol.
Ans: (a) What are alcohols? State their sources.
Alcohols are a class of organic compounds characterized by the presence of one or more hydroxyl (–OH) groups attached to a carbon atom.
Sources:
Natural Sources: Produced by the fermentation of sugars by yeast (e.g., ethanol in wine, beer).
Synthetic Sources: Manufactured industrially from petroleum products, primarily by the hydration of alkenes.
(b) Give general formulae of monohydric alcohol.
A monohydric alcohol contains only one hydroxyl group.
General Formula: CₙH₂ₙ₊₁OH or CₙH₂ₙO (where *n* = 1, 2, 3…)
Alkyl Group Formula: R–OH (where R is an alkyl group).
2. Give the :
(a) dot diagram
(b) abbreviated formula
(c) structure of second member of the alcohol group
(d) structure of alcohol with 4 carbon atoms
Ans: (a) Dot diagram
The dot diagram (electron dot structure) for the general functional group of alcohol (-OH) is:
H
• •
R : O :
• •
(b) Abbreviated formula
The general abbreviated formula for the alcohol group is R–OH, where ‘R’ represents any alkyl group.
(c) Structure of second member of the alcohol group
The second member has 2 carbon atoms. Its structure is:
H H
| |
H–C–C–O–H
| |
H H
Its IUPAC name is ethanol.
(d) Structure of alcohol with 4 carbon atoms
An alcohol with 4 carbon atoms can have different structures. The straight-chain structure (Butan-1-ol) is:
H H H H
| | | |
H–C–C–C–C–O–H
| | | |
H H H H
3. State the method of preparation of ethanol :
(a) by hydrolysis of ethene
(b) by hydrolysis of ethyl bromide
Ans: (a) By hydrolysis of ethene
Ethene is hydrated by heating with steam at high temperature (300°C) and high pressure (60–70 atm) in the presence of a catalyst like phosphoric acid.
CH2=CH2+H2O→CH3CH2OH
(b) By hydrolysis of ethyl bromide
Ethyl bromide is hydrolyzed by boiling with an aqueous solution of sodium hydroxide or potassium hydroxide.
CH3CH2Br+KOH(aq)→CH3CH2OH+KBr
4. Halo alkanes reacts with alkalies to produce alcohol. Give the equation for the preparation of second member of homologous series of alcohol. State under what condition the reaction occurs.
Ans: The second member of the homologous series of alcohols is Propan-1-ol.
The general equation for the preparation of an alcohol from a haloalkane is:
Haloalkane + Potassium Hydroxide → Alcohol + Potassium Halide
For the preparation of propan-1-ol, we start with the corresponding haloalkane, which is 1-chloropropane. The balanced chemical equation is:
CH₃-CH₂-CH₂-Cl + KOH (aq) → CH₃-CH₂-CH₂-OH + KCl
(1-Chloropropane) (Propan-1-ol)
Condition for the Reaction:
This reaction occurs under aqueous (water-based) and warm conditions.
The potassium hydroxide must be dissolved in water (aqueous KOH). The water provides the hydroxyl (OH⁻) ion that acts as a nucleophile, which attacks the carbon atom of the haloalkane, leading to the substitution of the chlorine atom and the formation of the alcohol. Gentle heating is often applied to increase the rate of the reaction.
5. (a) How do the boiling point and melting point change in the homologous series of alcohols?
(b) Name the product formed when ethanol reacts with acetic acid. Give an equation.
(c) What is the name given to this type of reaction?
Ans: (a) Boiling and Melting Points in Alcohols
In alcohols, both boiling and melting points rise as the molecular mass increases. This happens because longer carbon chains lead to stronger intermolecular forces, specifically more extensive hydrogen bonding and greater van der Waals attractions.
(b) Reaction of Ethanol with Acetic Acid
When ethanol reacts with acetic acid, an ester called ethyl acetate is formed.
Chemical Equation:
CH₃COOH + C₂H₅OH → CH₃COOC₂H₅ + H₂O
(c) Type of Reaction
This chemical change is classified as an esterification reaction.
6. Complete and balance the following equations. State the conditions wherever necessary.
(a) CH2=CH2+H2→………………
(b) C2H4+Cl2→………………
(c) C2H4+HCl→ ………………
(d) CaC2+H2O→ ………………
(e) C2H2+Br2→………………
(f) C2H5OH→K2Cr2O7 ………………
Ans:
(a) CH₂=CH₂ + H₂ → CH₃–CH₃
Condition: Catalyst (Ni, Pt, or Pd) and heat.
(b) C₂H₄ + Cl₂ → Cl–CH₂–CH₂–Cl
(Forms 1,2-dichloroethane)
(c) C₂H₄ + HCl → CH₃–CH₂Cl
(Forms chloroethane)
(d) CaC₂ + 2H₂O → Ca(OH)₂ + C₂H₂↑
(Acetylene gas is evolved)
(e) C₂H₂ + 2Br₂ → CHBr₂–CHBr₂
(Forms 1,1,2,2-tetrabromoethane)
Note: Reaction can occur in steps (first to CHBr=CHBr).
(f) C₂H₅OH → CH₃COOH
Condition: Acidified K₂Cr₂O₇ and heat.
(Balanced: 3C₂H₅OH + 2K₂Cr₂O₇ + 8H₂SO₄ → 3CH₃COOH + 2Cr₂(SO₄)₃ + 2K₂SO₄ + 11H₂O)
In short, ethanol oxidizes to ethanoic acid.
7. What is the effect of ethanol on human body?
Ans:Ethanol, the alcohol in drinks, slows down the brain, impairing judgment and coordination.
The liver works hard to process it, which can cause long-term damage. It can also raise blood pressure, irritate the stomach, and increase cancer risk.
Short-term, it causes intoxication. Long-term heavy use can lead to addiction and serious health issues like liver cirrhosis.
8. How are the following obtained :
(a) absolute alcohol
(b) spurious alcohol
(c) methylated spirit
Ans: (a) Absolute Alcohol
Absolute alcohol (nearly 100% pure ethanol) is obtained by removing the last 4-5% of water from ordinary rectified spirit (95.5% ethanol). This is typically done by a process called azeotropic distillation, where a water-removing agent like benzene or toluene is added. The added substance forms a low-boiling mixture with the water, allowing the pure, anhydrous ethanol to be collected.
(b) Spurious Alcohol
Spurious alcohol, also known as illicit or counterfeit liquor, is not obtained through a standardized process. It is illegally produced in unregulated, makeshift distilleries. It is often made by poorly fermenting a mixture of cheap, readily available sources like molasses, fruits, or grains, followed by crude and unhygienic distillation. To increase potency and volume, it is often dangerously adulterated with toxic substances like methanol.
(c) Methylated Spirit
Methylated spirit, also known as denatured alcohol, is obtained by mixing approximately 95% ethanol with poisonous or bad-tasting substances. Common additives include about 10% methanol (which makes it toxic), pyridine (which gives it a foul smell), and a violet or blue dye. This process is done to make the ethanol unfit for drinking, thereby allowing it to be sold for industrial or fuel purposes without the high taxes applied to potable alcohol.
9. Name the products formed and give appropriate chemical equations for the following :
(a) Sodium reacting with ethyl alcohol
(b) Ethanol oxidised by acidified potassium dichromate
Ans: (a) Sodium reacting with ethyl alcohol
Products: Sodium ethoxide and Hydrogen gas.
Chemical Equation:
2C2H5OH+2Na→2C2H5ONa+H2↑
(b) Ethanol oxidised by acidified potassium dichromate
Products: Acetaldehyde (which can further oxidise to Acetic acid).
Chemical Equation:
C2H5OH→CH3CHO+H2O
(With excess oxidising agent,
CH3CHO→CH3COOH
10. Give the trivial (common) names and the IUPAC names of the following :
(a) C2H2
(b) C2H4
(c) C2H6
(d) CH3OH
(e) C2H5OH
Ans: (a) C₂H₂
Trivial Name: Acetylene
IUPAC Name: Ethyne
(b) C₂H₄
Trivial Name: Ethylene
IUPAC Name: Ethene
(c) C₂H₆
Trivial Name: Ethyl hydride or Dimethyl
IUPAC Name: Ethane
(d) CH₃OH
Trivial Name: Methyl alcohol or Wood alcohol
IUPAC Name: Methanol
(e) C₂H₅OH
Trivial Name: Ethyl alcohol or Grain alcohol
IUPAC Name: Ethanol
11. Ethanol can be oxidised to ethanoic acid. Write the equation and name the oxidising agent.
Ans: Equation:
CH₃CH₂OH + 2[O] → CH₃COOH + H₂O
Oxidising agent: Acidified potassium dichromate(VI) (K₂Cr₂O₇/H₂SO₄)
12. Name an organic compound which is :
(a) used for illuminating country houses
(b) used for making a household plastic material
(c) called “wood spirit”
(d) poisonous and contains OH group
(e) consumed as a drink
(f) made from water gas
(g) solvent for ethanol
Ans: a) Methane – Used for lighting in country houses via gas lamps.
(b) Ethene – Used to make polyethylene plastic for household items.
(c) Methanol – Known as “wood spirit,” originally obtained from wood.
(d) Methanol – Contains an -OH group but is highly poisonous.
(e) Ethanol – The type of alcohol consumed in drinks.
(f) Methanol – Produced industrially from water gas (CO + H₂).
(g) Diethyl ether – A common solvent that can dissolve ethanol.
EXERCISE 12F
1. What are carboxylic acids? Give their general formula.
Ans: Carboxylic acids are a very important and common class of organic compounds. They are best known for being the chemical group that gives substances like vinegar (acetic acid) and citric fruits (citric acid) their sour taste and sharp smell.
In simple terms, a carboxylic acid is an organic molecule that contains the carboxyl functional group. This group is made up of a carbonyl group (a carbon atom double-bonded to an oxygen atom, C=O) and a hydroxyl group (-OH) both attached to the same carbon atom.
The general formula for a homologous series of carboxylic acids, where the molecules differ by a -CH₂- unit, is:CₙH₂ₙ₊₁COOH
Sometimes, the formula is also written as R-COOH, where ‘R’ represents any alkyl group (like -CH₃ for methyl, -C₂H₅ for ethyl, etc.).
2. Write the common name, IUPAC name and formula of one monocarboxylic acid and one dicarboxylic acid.
Ans:
1. Monocarboxylic Acid
A monocarboxylic acid has one carboxyl (-COOH) functional group in its molecule.
Common Name: Acetic Acid
This is the acid that gives vinegar its sour taste and pungent smell.
IUPAC Name: Ethanoic Acid
The IUPAC name is derived from the parent alkane, ethane. The ‘-e’ of ethane is replaced with ‘-oic acid’ to indicate the carboxylic acid functional group.
Formula: CH₃COOH
This structural formula shows the methyl group (CH₃-) attached to the carboxyl group (-COOH).
2. Dicarboxylic Acid
A dicarboxylic acid has two carboxyl (-COOH) functional groups in its molecule.
Common Name: Oxalic Acid
This acid is found naturally in many plants, like rhubarb and spinach.
IUPAC Name: Ethanedioic Acid
The IUPAC name is based on the parent alkane, ethane. The ‘-e’ is replaced with ‘-dioic acid’ to show the presence of two carboxylic acid groups.
Formula: HOOC-COOH
This formula clearly shows the two carboxyl groups connected to each other. It can also be written as (COOH)₂.
3. Write the names of :
(a) first three members of carboxylic acid series
(b) three compounds which can be oxidised directly, or in stages to produce acetic acid
Ans: (a) First three members of carboxylic acid series
The carboxylic acid series is characterized by the presence of the -COOH functional group. The first three members are named based on the number of carbon atoms they contain.
Methanoic acid (HCOOH) – Commonly known as formic acid, it is the simplest carboxylic acid.
Ethanoic acid (CH₃COOH) – Widely known as acetic acid, it is the main component of vinegar.
Propanoic acid (C₂H₅COOH) – This is the next acid in the series with three carbon atoms.
(b)Acetic acid (CH₃COOH) can be formed by the oxidation of various organic compounds. The oxidation can be a single-step process or may occur through intermediate stages.
Ethanol (CH₃CH₂OH): Ethanol, which is drinking alcohol, can be oxidized directly to acetic acid. This is the process behind vinegar turning sour.
Acetaldehyde (CH₃CHO): Acetaldehyde is an intermediate compound that is often formed when ethanol is oxidized in stages. It is then further oxidized to produce acetic acid.
Methanol (CH₃OH) and Carbon Monoxide (CO): In an industrial setting, acetic acid is produced by the direct combination of methanol and carbon monoxide through a catalytic process. This is a key commercial method for its production.
4. Vinegar is greyish in colour with a particular taste. Explain.
Ans: Vinegar looks greyish because it’s unfiltered. Tiny bits of raw material, such as rice or wood, are left in it from the fermentation process.Its sour taste comes from acetic acid. This acid is created when bacteria ferment the natural sugars in fruits or grains, giving vinegar its sharp, tangy flavor.
5. Give the structural formulae and IUPAC name of acetic acid. What is glacial acetic acid?
Ans: Structural Formula:
CH₃–COOH
IUPAC Name:
Ethanoic acid
Glacial Acetic Acid:
It is pure, concentrated ethanoic acid (about 99-100%) that freezes into ice-like crystals at temperatures below 16.6 °C, which is why it is called “glacial”.
6. Complete :
(a) Vinegar is prepared by the bacterial oxidation of ______
(b) The organic acid present in vinegar is ______
(c) The next higher homologue of ethanoic acid is ______
Ans: (a) Vinegar is prepared by the bacterial oxidation of ethanol or ethyl alcohol.
(b) The organic acid present in vinegar is ethanoic acid (which is also commonly called acetic acid).
(c) The next higher homologue of ethanoic acid is propanoic acid.
In part (a), the process involves specific bacteria (Acetobacter) that use oxygen from the air to convert the ethanol present in alcoholic liquids like wine or cider into acid, thus forming vinegar.
For part (b), the IUPAC name for the acid in vinegar is ethanoic acid, but its common name, acetic acid, is very widely used.
In part (c), homologues are compounds in the same series that differ by a -CH₂- unit. Ethanoic acid has two carbon atoms; adding a -CH₂- group gives a three-carbon acid, which is propanoic acid.
7. How is acetic acid prepared from
(a) ethanol
(b) acetylene?
Ans: (a) From Ethanol:
Ethanol is oxidized in the presence of an oxidizing agent like acidified potassium dichromate (K₂Cr₂O₇/H⁺) to form acetic acid.
CH3CH2OH→CH3CHO→CH3COOH
(b) From Acetylene:
Acetylene is first passed through a hot sulfuric acid solution containing mercuric sulfate (HgSO₄) catalyst to form acetaldehyde. The acetaldehyde is then oxidized (using an oxidizing agent like acidified K₂Cr₂O₇) to form acetic acid.
CH≡CH+H2O→CH3CHO→CH3COOH
8. What do you notice when acetic acid reacts with
(a) litmus
(b) metals
(c) alkalies
(d) alcohol?
Ans: (a) With litmus:
Turns blue litmus red, confirming it is acidic.
(b) With metals (e.g., zinc):
Produces hydrogen gas, which burns with a ‘pop’ sound.
(c) With alkalies (e.g., sodium hydroxide):
Forms salt and water in a neutralization reaction.
(d) With alcohol (e.g., ethanol):
When warmed with concentrated sulfuric acid, forms a sweet-smelling ester (ethyl acetate).
9. Acetic acid is a typical acid. Write one equation in each case for its reaction with
(a) a metal
(b) a base/alkali
(c) a carbonate
(d) a bicarbonate
Ans:
(a) With a metal
2CH3COOH+2Na→2CH3COONa+H2
(b) With a base/alkali
CH3COOH+NaOH→CH3COONa+H2O
(c) With a carbonate
2CH3COOH+Na2CO3→2CH3COONa+H2O+CO2
(d) With a bicarbonate
CH3COOH+NaHCO3→CH3COONa+H2O+CO2
MISCELLANEOUS QUESTIONS
1. (a) Write an equation for the laboratory preparation of
(i) an unsaturated hydrocarbon from calcium carbide
(ii) an alcohol from ethyl bromide
(b) What would you see, when ethyne is bubbled through a solution of bromine in carbon tetrachloride?
(c) Name the addition product formed between ethene and water
Ans: 1. (a)(i)CaC2+2H2O→C2H2+Ca(OH)2
(Ethyne is the unsaturated hydrocarbon.)
(ii)C2H5Br+KOH (aq)→C2H5OH+KBr
(Ethyl alcohol is formed.)
(b)The red-brown colour of bromine in carbon tetrachloride gets decolourised when ethyne is bubbled through it.
(c)Ethanol (or ethyl alcohol).
2. Ethanol can be converted into ethene which can be changed into ethane. Choose the correct word or phrase from the brackets to complete the following sentences:
(a) The conversion of ethanol into ethene is an example of ______ (dehydration, dehydrogenation)
(b) Converting ethanol into ethene requires the use of ______ (conc. HCl, conc. HNO₃, conc. H₂SO₄)
(c) The conversion of ethene into ethane is an example of ______ (hydration, hydrogenation)
(d) The catalyst used in the conversion of ethene into ethane is commonly ______ (iron, nickel, cobalt)
Ans: (a) dehydration
(b) conc. H₂SO₄
(c) hydrogenation
(d) nickel
3. Give reasons:
(a) Ethyne is more reactive than ethene
(b) Ethene is more reactive than ethane
(c) Hydrocarbons are excellent fuels
Ans: (a) Ethyne is more reactive than ethene
Ethyne has a triple bond (C≡C), while ethene has a double bond (C=C). The triple bond has two pi-bonds, which are weaker and more exposed, making them more easily available for addition reactions compared to the one pi-bond in ethene.
(b) Ethene is more reactive than ethane
Ethene has a double bond containing a reactive pi-bond, which readily undergoes addition reactions. Ethane only has strong, single sigma (σ) bonds, making it relatively inert and capable only of substitution reactions, which require more severe conditions.
(c) Hydrocarbons are excellent fuels
Hydrocarbons make excellent fuels because their combustion reaction with oxygen is highly exothermic, releasing a large amount of heat energy. They also burn with a clean flame, producing mainly CO₂ and H₂O, and are readily available from natural sources like petroleum and natural gas.
4. (a) Write balanced equations:
(i) when butane is burnt in oxygen
(ii) preparation of ethylene from ethyl alcohol
Ans: (i)2 C₄H₁₀ + 13 O₂ → 8 CO₂ + 10 H₂O
(ii)C₂H₅OH → C₂H₄ + H₂O
(Conc. H₂SO₄, 170°C)
5. Write the equations for the following lab. preparations:
(a) Ethane from sodium propionate
(b) Ethene from iodoethane
(c) Ethyne from calcium carbide
(d) Methanol from iodomethane
Ans:
(a) Ethane from sodium propionate
CH₃–CH₂–COONa + NaOH → CH₃–CH₃ + Na₂CO₃
(b) Ethene from iodoethane
CH₃–CH₂I + KOH (alc.) → CH₂=CH₂ + KI + H₂O
(c) Ethyne from calcium carbide
CaC₂ + 2H₂O → CH≡CH + Ca(OH)₂
(d) Methanol from iodomethane
CH₃I + NaOH (aq) → CH₃OH + NaI
6. (a) Write the equation for the preparation of carbon tetrachloride from methane
(b) Draw the structural formula of ethyne
(c) How is the structure of alkynes different from that of alkenes?
Ans:(a)Carbon tetrachloride is prepared from methane by chlorination in excess chlorine:
CH4+4Cl2→CCl4+4HCl
(b)Structural formula of ethyne (C₂H₂):
H─C≡C─H
H─C≡C─H
(c)Alkynes have at least one triple bond between two carbon atoms, while alkenes have a double bond.
This makes alkynes linear around the triple bond, whereas alkenes are planar but not fully linear.
7. Fill in the blanks with the correct words from the brackets:
Alkenes are the (a) …… (analogous/homologous) series of (b) …… (saturated/unsaturated) hydrocarbons. They differ from alkanes due to the presence of (c) …… (double/single) bonds. Alkenes mainly undergo (d) …… (addition/substitution) reactions.
Ans: (a) homologous
(b) unsaturated
(c) double
(d) addition
8. (a) Draw the structural formulae of the two isomers of Butane. Give the correct IUPAC name of each isomer
(b) State one use of acetylene
Ans: (a) Isomers of Butane
Structural Formula: CH₃-CH₂-CH₂-CH₃
IUPAC Name: Butane
Structural Formula: CH₃-CH(CH₃)-CH₃
IUPAC Name: 2-Methylpropane
(b)Acetylene is vital in metalworking for oxy-acetylene welding and cutting. Its primary benefit is the intensely hot flame—around 3,500°C—created when mixed with oxygen, making it ideal for steel.For welding, the flame melts metal edges, fusing them together, often with a filler rod for strength. For cutting, the flame preheats the steel before a jet of pure oxygen rapidly oxidizes and blows away the metal.Acetylene is also favored for its stable, controllable flame, which enhances safety. This powerful combination of extreme heat, precision, and effectiveness keeps it a fundamental tool in workshops and on construction sites globally.
9. Copy and complete the following table which relates to three homologous series of hydrocarbons:
Ans:
| General Formula | C6H2n | CnH2n-2 | CnH2n+2 |
| IUPAC name of the homologous series | Alkenes | Alkynes | Alkanes |
| Characteristic bond type | Double Bond | Triple Bond | Single Bonds |
| IUPAC name of the first member of the series | Ethene | Ethyne | Methane |
| Type of reaction with chlorine | Addition | Addition | Subtitution |
2008
(a) Name the organic compound prepared by each of the following reactions:
(i) C2H5COONa+NaOH→
(ii) CH3I+2H→
(iii) C2H5Br+KOH(alcoholic solution) →
(iv) CO+2H2 (Zinc oxide catalyst) →
(v) CaC2+2H2O→
(b) Write the equations for the following reactions:
(i) Calcium carbide and water
(ii) Ethene and water (steam)
(iii) Bromoethane and an aqueous solution of sodium hydroxide
(c) Distinguish between the saturated hydrocarbon ethane and the unsaturated hydrocarbon ethene by drawing their structural formulae
(d) Addition reactions and substitution reactions are types of organic reactions. Which type of reaction is shown by:
(i) ethane (ii) ethene
(e) (i) Write the equation for the complete combustion of ethane
(ii) Using appropriate catalysts, ethane can be oxidized to an alcohol, an aldehyde and an acid. Name the alcohol, aldehyde and acid formed when ethane is oxidized
(f) (i) Why is pure acetic acid known as glacial acetic acid?
(ii) What type of compound is formed by the reaction between acetic acid and an alcohol?
Ans: (a) Name the organic compound prepared from each reaction:
(i) Chloroethane (C₂H₅Cl) is prepared from ethane.
(ii) Ethanol (C₂H₅OH) is prepared from ethene.
(iii) Ethane (C₂H₆) is prepared from ethene.
(iv) Ethanoic Acid (CH₃COOH) is prepared from methanol.
(v) Ethane (C₂H₆) or Ethene (C₂H₄) is prepared from ethyne (depending on the amount of hydrogen used).
(b) Write the chemical equations for the following reactions:
(i) Preparation of Ethyne from Calcium Carbide:
CaC₂ + 2H₂O → C₂H₂ + Ca(OH)₂
(ii) Conversion of Ethene to Ethanol:
CH₂=CH₂ + H₂O → CH₃-CH₂OH
(This reaction requires a catalyst like phosphoric acid and heat.)
(iii) Conversion of Bromoethane to Ethanol:
CH₃-CH₂Br + NaOH (aq) → CH₃-CH₂OH + NaBr
(This is a nucleophilic substitution reaction.)
(c) How would you distinguish chemically between Ethane and Ethene?
The easiest way to tell ethane and ethene apart is by using a test for unsaturation, like bromine water.Ethene contains a carbon-carbon double bond, which is unsaturated. If you bubble ethene gas through red-brown bromine water, it will decolorize the bromine water quickly. This happens because ethene reacts with bromine in an addition reaction.Ethane is a saturated hydrocarbon with only single bonds. If you bubble ethane gas through the same bromine water, no reaction occurs, and the red-brown colour remains unchanged.
(d) Name the type of reaction that is characteristic of:
(i) Ethane: Substitution Reaction.
> Ethane typically undergoes substitution reactions. For example, in the presence of sunlight, it reacts with chlorine, where chlorine atoms replace hydrogen atoms one at a time.
(ii) Ethene: Addition Reaction.
> Ethene is known for its addition reactions. Due to its double bond, it can add molecules like hydrogen (to form ethane), bromine, or water (to form ethanol) across the double bond.
(e) What happens when Ethane is oxidized?
The products of ethane’s oxidation depend on the conditions:
Complete Combustion (in excess oxygen):
When ethane burns completely, it undergoes full oxidation to produce carbon dioxide and water, releasing a lot of heat and energy.
2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O
Controlled or Incomplete Oxidation:
Under specific controlled conditions with limited oxygen or specific oxidizing agents, ethane can be oxidized in steps to form different useful compounds:
It can first form Ethanol (an alcohol).
Further oxidation can produce Ethanal (an aldehyde).
Ultimately, it can be oxidized to Ethanoic Acid (acetic acid).
(f) Answer the following about Acetic Acid:
(i) Why is pure acetic acid called glacial acetic acid?
> Pure acetic acid has a freezing point of about 16.6°C, which is just below room temperature. When it is cooled, it solidifies into colorless, ice-like crystals. This resemblance to ice (glaciers) is why it is called “glacial” acetic acid.
(ii) What is formed when acetic acid reacts with ethanol in the presence of an acid catalyst?
> When acetic acid and ethanol are heated together with a concentrated acid catalyst (like concentrated sulphuric acid), a sweet-smelling ester is formed. The specific compound produced is Ethyl Ethanoate (commonly known as ethyl acetate).
> CH₃COOH + CH₃CH₂OH → CH₃COOCH₂CH₃ + H₂O
2009
(a) Which of the following statements is wrong about alkanes?
(i) They are all saturated hydrocarbon
(ii) They can undergo addition as well as substitution reaction
(iii) They are almost non polar in nature
(iv) On complete combustion give out carbon dioxide and water
(b) The organic compound obtained as the end product of the fermentation of sugar solution is:
(i) Methanol (ii) Ethanol (iii) Ethane (iv) Methanoic acid
(c) Find the odd one out and explain:
C3H6,C3H8,C2H6,CH4
(d) Give chemical equation for:
(i) The laboratory preparation of methane from sodium acetate
(ii) The industrial preparation of methanol from water gas
(iii) The reaction of one mole of ethene with one mole of chlorine gas
(iv) The preparation of ethylene from 1,2-dibromoethane
(e) State how the following conversions can be carried out:
(i) Ethyl chloride to Ethyl alcohol
(ii) Ethyl chloride to Ethene
(iii) Ethene to Ethyl alcohol
(iv) Ethyl alcohol to Ethene
(f) (i) Define isomerism
(ii) Give the IUPAC name of the isomer C4H10 which has a branched chain
Ans: (a) (ii) They can undergo addition as well as substitution reaction.
Explanation: Alkanes are saturated hydrocarbons and only undergo substitution reactions. They do not undergo addition reactions.
(b) The organic compound obtained as the end product of the fermentation of sugar solution is:
Answer: (ii) Ethanol
(c) Find the odd one out and explain: C₃H₆, C₃H₈, C₂H₆, CH₄
Answer: C₃H₆ is the odd one out.
Explanation: C₃H₈, C₂H₆, and CH₄ are all alkanes (saturated hydrocarbons with the general formula CₙH₂ₙ₊₂). C₃H₆ is an alkene (unsaturated with the general formula CₙH₂ₙ).
(d) Give chemical equation for:
(i) Laboratory preparation of methane from sodium acetate:
CH₃COONa + NaOH (CaO/Δ) → CH₄ + Na₂CO₃
(ii) Industrial preparation of methanol from water gas:
CO + 2H₂ (ZnO-Cr₂O₃/200-300 atm/300°C) → CH₃OH
(iii) Reaction of one mole of ethene with one mole of chlorine gas:
CH₂=CH₂ + Cl₂ → Cl-CH₂-CH₂-Cl (1,2-dichloroethane)
(iv) Preparation of ethylene from 1,2-dibromoethane:
CH₂Br-CH₂Br + Zn → CH₂=CH₂ + ZnBr₂
(e) State how the following conversions can be carried out:
(i) Ethyl chloride to Ethyl alcohol: Nucleophilic substitution with aqueous KOH.
CH₃-CH₂-Cl + KOH(aq) → CH₃-CH₂-OH + KCl
(ii) Ethyl chloride to Ethene: Dehydrohalogenation with alcoholic KOH.
CH₃-CH₂-Cl + KOH(alcohol) → CH₂=CH₂ + KCl + H₂O
(iii) Ethene to Ethyl alcohol: Direct hydration by adding steam in the presence of an acid catalyst (H₃PO₄).
CH₂=CH₂ + H₂O (H₃PO₄/300°C/60-70 atm) → CH₃-CH₂-OH
(iv) Ethyl alcohol to Ethene: Dehydration with concentrated H₂SO₄ at 170°C.
CH₃-CH₂-OH (Conc. H₂SO₄/170°C) → CH₂=CH₂ + H₂O
(f)(i) Define isomerism: Isomerism is a phenomenon where two or more organic compounds have the same molecular formula but different physical and chemical properties due to a different arrangement of atoms.
(ii) IUPAC name of the branched chain isomer of C₄H₁₀: 2-Methylpropane (Common name: Isobutane). Its structure is (CH₃)₂CH-CH₃.
2010
(a) An organic compound undergoes addition reactions and gives a red colour precipitate with ammoniacal cuprous chloride. Therefore, the organic compound could be:
(i) Ethane (ii) Ethene (iii) Ethyne (iv) Ethanol
(b) An organic weak acid is:
(i) Formic acid (ii) Sulphuric acid (iii) Nitric acid (iv) Hydrochloric acid
(c) The organic compound mixed with ethanol to make it spurious is:
(i) Methanol (ii) Methanoic acid (iii) Methanal (iv) Ethanoic acid
(d) Draw the structural formula for each of the following:
(i) Ethanoic acid (ii) But-2-yne
(e) Compound A is bubbled through bromine dissolved in carbon tetrachloride and the product is CH2Br–CH2Br
(i) Draw the structural formula of A
(ii) What type of reaction has A undergone?
(iii) What is your observation?
(iv) Name (not formula) the compound formed when steam reacts with A in the presence of phosphoric acid
(v) What is the procedure for converting the product of (e)(iv) back to A?
Ans:
(a) Organic compound giving red precipitate with ammoniacal cuprous chloride:
(iii) Ethyne
Reason: Only terminal alkynes like ethyne form red copper acetylide with ammoniacal Cu₂Cl₂.
(b) Organic weak acid:
(i) Formic acid
Reason: Formic acid (HCOOH) is a weak organic acid; others are strong inorganic acids.
(c) Compound mixed with ethanol to make it spurious:
(i) Methanol
Reason: Methanol is toxic and added illegally to ethanol, causing severe health risks.
(d) structural formula
(i) Ethanoic acid
O
║
H ─ C ─ C ─ OH
│
H
(ii) But-2-yne
H₃C ─ C ≡ C ─ CH₃
(e) (i)
H₂C=CH₂
(ii) Type of Reaction: Addition Reaction
The reaction described is a classic example of an Addition Reaction. Ethene (C₂H₄) is an unsaturated hydrocarbon with a carbon-carbon double bond. This double bond is an area of high electron density, making it susceptible to attack. In an addition reaction, a molecule like bromine (Br₂) adds directly across the double bond. The pi bond (the second bond of the double bond) breaks, and new single bonds are formed with the bromine atoms. This process saturates the molecule, turning it from an alkene into an alkane derivative.
(iii) Observation: Red-brown Bromine Colour Disappears
This is the tell-tale experimental observation that confirms the presence of a carbon-carbon double bond. Bromine water is a red-brown coloured solution. When it is added to ethene and shaken, the red-brown colour decolorizes (disappears) rapidly. The reason for this is that the bromine molecules (Br₂) are being consumed as they add to the ethene molecules to form a new, colourless compound called 1,2-dibromoethane. The disappearance of the colour is a simple, visual test for unsaturation.
(iv) Product with Steam: Ethanol (C₂H₅OH)
Ethene can be converted into ethanol through a process called hydration. This is an industrial method where ethene gas is mixed with steam (water vapour) and passed over a solid catalyst, commonly phosphoric acid (H₃PO₄), under high pressure and temperature (around 300°C and 60-70 atm). During this reaction, a water molecule adds across the double bond of ethene. The reaction can be represented as:
C₂H₄ (g) + H₂O (g) → C₂H₅OH (l)
(Ethene + Steam → Ethanol)
(v) Conversion Back to A: Heating Ethanol with Concentrated H₂SO₄ at 170°C
This is the reverse process, which converts the ethanol back into our original compound, ethene. This reaction is known as dehydration, as it involves the removal of a water molecule. When ethanol is heated to around 170°C with concentrated sulphuric acid acting as a dehydrating agent and a catalyst, a molecule of water is eliminated from the ethanol. This removal re-forms the carbon-carbon double bond, regenerating ethene gas.
C₂H₅OH (l) → C₂H₄ (g) + H₂O (g)
The specific temperature of 170°C is crucial because at a lower temperature (e.g., 140°C), a different reaction (intermolecular dehydration) occurs to form a different compound, diethyl ether.
2011
(a) The functional group present in acetic acid is:
(i) Ketonic >C=O
(ii) Hydroxyl –OH
(iii) Aldehydic –CHO
(iv) Carboxyl –COOH
(b) The unsaturated hydrocarbons undergo:
(i) a substitution reaction (ii) an oxidation reaction (iii) an addition reaction (iv) none of the above
(c) The number of C–H bonds in ethane molecule are:
(i) Four (ii) Six (iii) Eight (iv) Ten
(d) Choose the correct word/phrase from within the brackets to complete the following sentences:
(i) The catalyst used for conversion of ethene to ethane is commonly …… (nickel / iron / cobalt)
(ii) When acetaldehyde is oxidized with acidified potassium dichromate, it forms …… (ester / ethanol / acetic acid)
(iii) Ethanoic acid reacts with ethanol in presence of concentrated H2SO4, so as to form a compound and water. The chemical reaction which takes place is called …… (dehydration / hydrogenation / esterification)
(iv) Write the equation for the reaction taking place between 1,2-dibromoethane and alcoholic potassium hydroxide
(v) The product formed when ethene gas reacts with water in the presence of sulphuric acid …… (ethanol / ethanal / ethanoic acid)
(e) Write balanced chemical equations for the following:
(i) Monochloro ethane is hydrolysed with aqueous KOH
(ii) A mixture of sodalime and sodium acetate is heated
(iii) Ethanol under high pressure and low temperature is treated with acidified potassium dichromate
(iv) Water is added to calcium carbide
(v) Ethanol reacts with sodium at room temperature
Ans: (a) The functional group present in acetic acid is:
(iv) Carboxyl –COOH
Acetic acid (CH₃COOH) is a carboxylic acid. Its defining functional group is the carboxyl group, which is a combination of a carbonyl (>C=O) and a hydroxyl (–OH) group, represented as –COOH.
(b) The unsaturated hydrocarbons undergo:
(iii) an addition reaction
Unsaturated hydrocarbons, which contain double or triple bonds (e.g., alkenes and alkynes), have electron-rich areas that allow other atoms or groups to add across the multiple bond. This is characteristic of an addition reaction.
(c) The number of C–H bonds in an ethane molecule are:
(ii) Six
The molecular formula of ethane is C₂H₆. This means each molecule consists of two carbon atoms and six hydrogen atoms. Each hydrogen atom is connected to a carbon atom by a single bond, so there are six C–H bonds in total.
(d) Complete the following sentences:
(i)Ethene (C₂H₄) is converted to ethane (C₂H₆) by the addition of hydrogen gas (H₂). This hydrogenation reaction is commonly facilitated by a nickel catalyst.
(ii)Acidified potassium dichromate is a strong oxidizing agent. It oxidizes acetaldehyde (an aldehyde, CH₃CHO) to acetic acid (a carboxylic acid, CH₃COOH).
(iii) Ethanoic acid reacts with ethanol in the presence of concentrated H₂SO₄, so as to form a compound and water.
The reaction between a carboxylic acid and an alcohol in the presence of an acid catalyst produces an ester and water. This specific reaction is known as esterification.
(iv) Write the equation for the reaction taking place between 1,2-dibromoethane and alcoholic potassium hydroxide.
CH2Br−CH2Br+2KOH (alcoholic)→CH≡CH+2KBr+2H2O
Explanation: Alcoholic potassium hydroxide (KOH) is a strong base that promotes elimination reactions. When it reacts with a vicinal dihalide like 1,2-dibromoethane, it results in the removal of two molecules of HBr, forming an alkyne (ethyne, commonly known as acetylene).
(v) The product formed when ethene gas reacts with water in the presence of sulphuric acid is ethanol.
This is a classic industrial method for preparing ethanol. Ethene undergoes an addition reaction with water (hydration) in the presence of an acid catalyst like sulfuric acid to form ethanol.
(e) Write balanced chemical equations for the following:
(i) Monochloroethane is hydrolyzed with aqueous KOH.
CH3−CH2Cl+KOH(aq)→CH3−CH2OH+KCl
Explanation: Aqueous KOH provides OH⁻ ions which act as a nucleophile, leading to a substitution reaction where the chlorine atom is replaced by a hydroxyl (–OH) group, forming ethanol.
(ii)CH3COONa+NaOH(sodalime)→CH4+Na2CO3
Explanation: This is the decarboxylation reaction for preparing alkanes. Soda lime (a mixture of NaOH and CaO) when heated with a sodium salt of a carboxylic acid, removes a molecule of CO₂, resulting in an alkane with one less carbon atom. Here, methane (CH₄) is produced.
(iii) Ethanol under high pressure and low temperature is treated with acidified potassium dichromate.
CH3CH2OH→H+CH3CHO
Explanation: Acidified K₂Cr₂O₇ is an oxidizing agent. Under controlled, mild conditions (low temperature and high pressure), the oxidation of ethanol can be stopped at the aldehyde stage, producing ethanal (acetaldehyde). Under vigorous conditions, it would proceed to acetic acid.
(iv) Water is added to calcium carbide.
CaC2+2H2O→Ca(OH)2+C2H2
Explanation: This is a standard laboratory preparation for ethyne (acetylene) gas. Calcium carbide reacts vigorously with water to produce calcium hydroxide and ethyne gas.
(v) Ethanol reacts with sodium at room temperature.
2CH3CH2O+2Na→2CH3CH2ONa+H2
Explanation: Ethanol, like other alcohols, has an acidic hydrogen attached to the oxygen. It reacts with reactive metals like sodium to liberate hydrogen gas and form the sodium salt of the alcohol, called sodium ethoxide.
2012
(a) Give the structural formula for the following:
(i) Methanoic acid (ii) Ethanal (iii) Ethyne (iv) Acetone (v) 2-methyl propane
(b) From the following organic compounds given below, choose one compound in each case which relates to the description [i] to [iv]:
[Ethyne, ethanol, acetic acid, ethene, methane]
(i) An unsaturated hydrocarbon used for welding purposes
(ii) An organic compound whose functional group is carboxyl
(iii) A hydrocarbon which on catalytic hydrogenation gives a saturated hydrocarbon
(iv) An organic compound used as a thermometric liquid
(c) (i) Why is pure acetic acid known as glacial acetic acid?
(ii) Give a chemical equation for the reaction between ethyl alcohol and acetic acid
Ans: (a) Structural Formulae
(i) Methanoic acid: H–COOH
(ii) Ethanal: CH₃–CHO
(iii) Ethyne: H–C≡C–H
(iv) Acetone: CH₃–CO–CH₃
(v) 2-methyl propane: CH₃–CH(CH₃)–CH₃
(b) Compound Identification
(i) Ethyne – Used in welding torches.
(ii) Acetic acid – Has the carboxyl (–COOH) group.
(iii) Ethene – Forms ethane (C₂H₆) upon hydrogenation.
(iv) Ethanol – Commonly used in thermometers.
(c) Explanations and Equation
(i) Pure acetic acid is called glacial acetic acid because it solidifies into ice-like crystals at 16.6°C.
(ii) Chemical Equation:
CH₃COOH + C₂H₅OH → CH₃COOC₂H₅ + H₂O
(Ethanol and acetic acid react to form ethyl acetate and water)
2013
(a) (i) Give a chemical test to distinguish ethene gas and ethane gas
(ii) Identify the statement that is incorrect about alkanes:
(A) They are hydrocarbons
(B) There is a single covalent bond between carbon and hydrogen
(C) They can undergo both substitution as well as addition reactions
(D) On complete combustion they produce carbon dioxide and water
(b) Give the structural formulae for the following:
(i) An isomer of n-butane
(ii) 2-propanol
Ans:
(a) (i) Chemical test to distinguish ethene and ethane:
Pass the gases separately through bromine water (reddish-brown).
Ethene: Decolorizes bromine water.
Ethane: Does not decolorize bromine water.
(a) (ii) Incorrect statement about alkanes:
(C) They can undergo both substitution as well as addition reactions
Alkanes undergo substitution reactions, but not addition reactions.
(b)(i) An isomer of n-butane
H H H
| | |
H – C – C – C – H
| | |
H C H
|
H
(ii) 2-propanol
H OH H
| | |
H – C – C – C – H
| | |
H H H
2014
(a) The I.U.P.A.C. name of acetylene is:
(i) propane (ii) propyne (iii) ethene (iv) ethyne
(b) Name hydrocarbons containing a –C–functional group
(c) Give preparation of ethane from sodium propionate
(d) Distinguish ethane and ethene (using alkaline potassium permanganate solution)
(e) Give the structural formula of the following:
(i) ethanol (ii) 1-propanal (iii) ethanoic acid (iv) 1,2-dichloroethane
(f) Give preparation of ethanol from monochloroethane and aq. sodium hydroxide
Ans:
(a) IUPAC name of acetylene
Ethyne
(b) Hydrocarbons with –C≡C– group
Alkynes
(c) Ethane from sodium propionate
On heating sodium propionate with soda-lime:
CH3CH2COONa+NaOH→CH3–CH3+Na2CO3
Decarboxylation removes CO₂, forming ethane.
(d) Distinguish ethane and ethene using alkaline KMnO₄
Ethene: Decolorizes purple KMnO₄.
Ethane: No decolorization; remains purple.
(e) Structural formulae
(i) Ethanol: CH₃–CH₂–OH
(ii) 1-Propanal: CH₃–CH₂–CHO
(iii) Ethanoic acid: CH₃–COOH
(iv) 1,2-Dichloroethane: Cl–CH₂–CH₂–Cl
(f) Ethanol from monochloroethane and aq. NaOH
Heating monochloroethane with aqueous NaOH gives ethanol via nucleophilic substitution:
CH3–CH2–Cl+NaOH (aq)→CH3–CH2–OH+NaCl