1. Basic Concept of a Spectrum
When a beam of composite light (like white light) is passed through a prism, it splits into its constituent colours.. White light produces a continuous band of seven colours, remembered by the acronym VIBGYOR (Violet, Indigo, Blue, Green, Yellow, Orange, Red).
2. Formation of a Spectrum
The splitting occurs due to two main phenomena:
Dispersion: This is the process of splitting white light into its constituent colours. It happens because different colours have different wavelengths and thus refract (bend) by different amounts when passing through a prism. Violet light has the shortest wavelength and bends the most, while red light has the longest wavelength and bends the least.
Deviation: The overall bending of the light ray from its original path is called deviation. Violet light suffers the greatest deviation, and red light suffers the least.
3. Types of Spectra
There are two main types:
Pure Spectrum: It is a spectrum in which the constituent colours are distinct and do not overlap. It is produced using a narrow slit and a lens to focus the light, resulting in a sharp, well-defined image of the slit for each colour.
Impure Spectrum: It is a spectrum where the colours are overlapping and not clearly separated. This happens if the source of light is broad, like a filament bulb, without using a proper narrow slit.
4. Recombination of White Light
Newton proved that dispersion is a reversible process. If the seven colours of the spectrum are made to fall on a second, identical prism placed in an inverted position, they recombine to form a beam of white light.
5. The Electromagnetic Spectrum
This is a much broader concept that includes the visible light spectrum. It is the entire range of electromagnetic waves arranged in order of their frequency or wavelength. The main regions, from longest wavelength (lowest frequency) to shortest wavelength (highest frequency), are:
Radio Waves: Used in communication (radio, TV).
Microwaves: Used in radar, microwave ovens, and mobile phones.
Infrared (IR) Rays: Felt as heat; used in remote controls, night-vision devices, and physiotherapy.
Visible Light: The only part detectable by the human eye.
Ultraviolet (UV) Rays: Causes suntan and sunburn; used for sterilizing purposes and in forensic labs.
X-rays: Can penetrate soft tissues but not bones; used in medical diagnosis.
Gamma Rays: Have the highest energy and penetrating power; used in cancer treatment (radiotherapy).
6. Key Scattering Phenomenon: Tyndall Effect
The scattering of light by colloidal particles in its path is called the Tyndall Effect. It makes the path of the light beam visible (e.g., a beam of sunlight entering a dusty room).
Why the Sky is Blue: The earth’s atmosphere scatters blue light (shorter wavelength) more strongly than red light. When we look at the sky, this scattered blue light reaches our eyes, making the sky appear blue.
Why the Sun appears Red during Sunrise/Sunset: At sunrise and sunset, sunlight travels through a thicker layer of the atmosphere. Most of the blue light is scattered away, and the longer wavelength red and orange light reaches our eyes, making the sun appear reddish.
EXERCISE 6(A)
Question 1:
Name three factors on which the deviation produces by a prism depends and state how it depends on the factors stated by you.
Ans: The deviation produced by a prism depends on the following three factors:
Angle of Prism (A): The deviation increases with an increase in the angle of the prism. A prism with a larger angle bends light more.
Angle of Incidence (i): The deviation first decreases, reaches a minimum value (minimum deviation), and then increases again as the angle of incidence changes.
Wavelength of Light (Refractive Index): The deviation is greater for light of shorter wavelengths (like blue light) than for longer wavelengths (like red light). This is because the refractive index of the prism material is higher for shorter wavelengths.
Question 2:
How does the deviation produced by a triangular prism depend on the colours (or wavelengths) of light incident on it?
Ans: When white light passes through a triangular prism, it splits into its constituent colours, a phenomenon known as dispersion. The deviation of light depends on its colour (or wavelength) in the following way:
Shorter wavelengths of light, like violet and blue, are deviated the most. This is because glass has a higher refractive index for these colours, causing them to bend more.
Longer wavelengths of light, like red and orange, are deviated the least. The refractive index of glass is lower for these colours, so they bend less.
Therefore, the angle of deviation is inversely related to the wavelength of light: higher deviation for shorter wavelengths and lower deviation for longer wavelengths.
Question 3:
Which colour of white light is deviated by a glass prism the most and which the least?
Ans: When a beam of white light passes through a glass prism, it splits into its constituent colours—a phenomenon known as dispersion. Among these colours, violet light is deviated the most, while red light is deviated the least.
This happens because different colours of light have different wavelengths, and the glass material of the prism refracts each colour by a different amount. Violet light has the shortest wavelength and experiences the greatest bending (maximum deviation), whereas red light, with the longest wavelength, is bent the least (minimum deviation).
Question 4:
Define the term dispersion of light.
Ans: Dispersion of light is the phenomenon in which white light splits into its seven constituent colors (VIBGYOR – Violet, Indigo, Blue, Green, Yellow, Orange, Red) when it passes through a transparent medium like a glass prism. This happens because different colors of light have different wavelengths and thus travel at different speeds in the medium, causing them to refract (bend) by different amounts. Violet light, having the shortest wavelength, bends the most, while red light, with the longest wavelength, bends the least. A common example of dispersion is the formation of a rainbow, where sunlight is dispersed by raindrops in the atmosphere.
Question 5:
Explain the cause of dispersion of white light through a prism.
Ans: When white light passes through a prism, it separates into its constituent colors, forming a spectrum (VIBGYOR). This occurs because white light is composed of different colors, each with a distinct wavelength.
Shorter wavelengths, such as violet, slow down more and bend more sharply. Longer wavelengths, like red, slow down less and bend less. This variation in bending, due to differences in wavelength, causes the light to spread out and create the colorful spectrum we observe.
Question 6:
Explain briefly, with the help of a neat labelled diagram, how white light gets dispersed by a prism.
Ans:
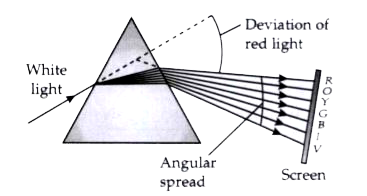
When a beam of white light passes through a prism, it gets dispersed into its constituent colours, forming a spectrum.
Explanation with diagram description:
Incident white light: A ray of white light (from the sun or a bulb) is made to fall on one surface of a triangular glass prism.
Refraction: As the light enters the prism, it bends towards the normal because it travels from air (rarer medium) to glass (denser medium).
Dispersion inside the prism: White light is made of different wavelengths. The prism refracts each colour by a different amount — violet bends the most, red the least.
Emergent ray: When these separated colours come out of the other side of the prism, they bend away from the normal (glass to air) and spread further into a band of colours (VIBGYOR: Violet, Indigo, Blue, Green, Yellow, Orange, Red).
Thus, the prism splits white light due to wavelength-dependent refraction — this is dispersion.
Question 7:
The diagram shown below in Fig. 6.11 shows the path taken by a narrow beam of yellow monochromatic light passing through an equiangular glass prism. Now the yellow light is replaced by a narrow beam of white light incident at the same angle. Draw another diagram to show the passage of white light through the prism and label it to show the effect of prism on the white light.
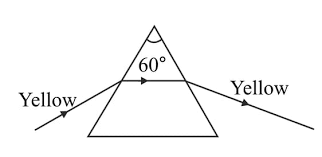
Ans:

When a beam of white light passes through a prism, it gets dispersed into its constituent colours, forming a spectrum.
Explanation with diagram description:
Incident white light: A ray of white light (from the sun or a bulb) is made to fall on one surface of a triangular glass prism.
Refraction: As the light enters the prism, it bends towards the normal because it travels from air (rarer medium) to glass (denser medium).
Dispersion inside the prism: White light is made of different wavelengths. The prism refracts each colour by a different amount — violet bends the most, red the least.
Emergent ray: When these separated colours come out of the other side of the prism, they bend away from the normal (glass to air) and spread further into a band of colours (VIBGYOR: Violet, Indigo, Blue, Green, Yellow, Orange, Red).
Thus, the prism splits white light due to wavelength-dependent refraction — this is dispersion
Question 8:
How does the speed of light in glass change on increasing the wavelength of light?
Ans: When the wavelength of light increases, the speed of light in glass also increases.
Explanation:
The speed of light in any transparent medium like glass is given by
v=c/n
, where
c is the speed of light in vacuum and
n is the refractive index of the medium.
The refractive index n of glass depends on the wavelength of light—it decreases as the wavelength increases (this is called normal dispersion).
Since
n becomes smaller for longer wavelengths, the value of
v=c/n becomes larger.
Therefore, increasing the wavelength reduces the refractive index, which in turn increases the speed of light in glass.
Question 9:
Which colour of white light travels (a) fastest (b) slowest, in glass?
Ans: (a) Fastest: Red light
(b) Slowest: Violet light
Explanation:
In glass, different colours of light travel at different speeds due to a property called dispersion. Red light has the longest wavelength and is bent the least, allowing it to travel fastest. Violet light has the shortest wavelength and is bent the most, causing it to travel slowest.
Question 10:
Fig. 6.12 shows a thin beam of white light from a source S striking on one face of a prism.
(a) Complete the diagram to show effect of prism on the beam and to show what is seen on the screen.
(b) A slit is placed in between the prism and the screen to pass only the light of green colour. What will you then observe on the screen?
(c) What conclusion do you draw from the observation in part (b) above?
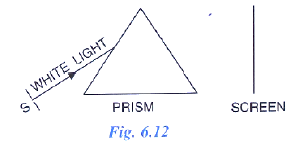
Ans: (a) The completed diagram shows a beam of white light entering the prism and emerging as a band of seven colours—Violet, Indigo, Blue, Green, Yellow, Orange, and Red—forming a spectrum on the screen.
(b) When a slit is placed to allow only green light through, a single sharp green band appears on the screen, while the other colours are blocked.
(c) This experiment shows that white light is made up of seven different colours. A prism splits white light into these colours, a process called dispersion. The green band is one of these pure colours.
Question 11:
(a) If a monochromatic beam of light undergoes minimum deviation through an equiangular prism, how does the beam pass through the prism, with respect to its base?
(b) If white light is used in same way as in part (a) above, what change is expected in the emergent beam?
(c) What conclusion do you draw about the nature of white light in part (b)?
Ans: (a) When a monochromatic beam undergoes minimum deviation in an equiangular prism, it passes parallel to the base of the prism.
(b) If white light is used in the same way, the emergent beam will split into a spectrum of colours (VIBGYOR) instead of remaining a single beam.
(c) This shows that white light is polychromatic (made up of several colours), and different colours travel at different speeds in the prism, causing dispersion.
Question 12:
What do you understand by the term spectrum?
Ans: The term spectrum refers to the band of coloured components obtained when light is split by a dispersion medium, such as a glass prism. When white light passes through a prism, it separates into its constituent colours—violet, indigo, blue, green, yellow, orange, and red—forming what is known as a visible spectrum. This ordered arrangement of colours demonstrates that white light is a mixture of different wavelengths, each bending by a different amount as they pass through the prism.
Question 13:
A ray of white light is passed through a glass prism and spectrum is obtained on a screen.
(a) Name the seven colours of the spectrum in order.
(b) Do the colours have the same width in the spectrum?
(c) Which of the colour of the spectrum of white light deviates (i) the most, (ii) the least?
Ans:
(a) When white light passes through a prism, it splits into a band of seven colours called a spectrum. The correct order of these colours, from the one that bends the most to the one that bends the least, is: Violet, Indigo, Blue, Green, Yellow, Orange, and Red. A simple way to remember this sequence is by using the acronym VIBGYOR.
(b) No, the colours in the spectrum do not have the same width. You might notice that some colours, like blue and green, appear to spread across a broader area, while others, such as indigo, seem much narrower. This happens because the prism disperses different colours by different amounts.
(c)
(i) The colour that deviates the most from its original path is Violet. This is because violet light has the shortest wavelength, so it slows down and bends more when it passes through the glass of the prism.
(ii) The colour that deviates the least is Red. Red light has the longest wavelength, so it is affected less by the prism and bends only slightly.
Question 14:
The wavelengths for the light of red and blue colours are roughly 7.8×107 m and 4.8×107 m respectively.
(a) Which colour has the greater speed in vacuum?
(b) Which colour has the greater speed in glass?
(a) Which colour has the greater speed in vacuum?
Ans: Both colours have the same speed in a vacuum. The speed of all electromagnetic waves, including all colours of light, is the same in a vacuum, approximately 3 × 10⁸ m/s.
(b) Which colour has the greater speed in glass?
Red light has a greater speed in glass. The speed of light in a medium depends on its wavelength; longer wavelengths (like red light) travel faster compared to shorter wavelengths (like blue light) when passing through a material like glass.
Question 15:
(i) Draw a diagram to show the splitting of white light by a prism into its constituent colour.
(ii) Draw another diagram to show how the colours of spectrum of white light can be combined to give the effect of white light.
Ans: (i) Diagram showing the splitting of white light by a prism:
Imagine a triangular glass prism. A ray of white light is shown entering one of the prism’s faces. As it passes through the prism, the light bends (refracts). However, each colour that makes up white light bends by a different amount. This causes the single ray of white light to spread out into a band of seven colours, called a spectrum, as it exits the prism. The order of colours is: Violet, Indigo, Blue, Green, Yellow, Orange, and Red (often remembered by the acronym VIBGYOR).
(ii) Diagram showing the recombination of colours to form white light:
To show the colours recombining, a second, identical prism is placed in an inverted position behind the first one. The spectrum of colours coming from the first prism is allowed to fall on this second prism. The second prism bends the colours again, but in the opposite way. All the different coloured rays are brought back together (recombined) and emerge from the second prism as a single ray of white light. This demonstrates that white light is a mixture of the seven colours of the spectrum.
Question 16:
Complete the ray diagram given below to show the nature of light produced on the screen.
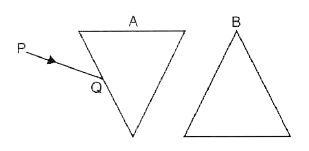
Ans: In the completed ray diagram for a convex lens:
A real and inverted image of the object is formed on the screen. This image is located between the focus (F) and the point 2F on the opposite side of the lens. Compared to the original object, the resulting image is diminished, meaning it is smaller in size.
Question 17:
Name the subjective property of light related to its wavelength.
Ans: Colour
Question 18:
What is the range of wavelength of the spectrum of white light in (i) Å, (ii) nm?
Ans: (i) In Ångström (Å): The range of wavelength for white light is approximately 4000 Å to 8000 Å.
(ii) In nanometres (nm): The range of wavelength for white light is approximately 400 nm to 800 nm.
Question 19:
Write the approximate wavelengths for (i) blue and (ii) red light.
Ans:
(i) The approximate wavelength for blue light is 480 nanometers.
(ii) The approximate wavelength for red light is 650 nanometers.
Explanation:
Visible light is a small part of the electromagnetic spectrum that our eyes can detect. Blue light has a relatively short wavelength, typically ranging from about 450 to 495 nanometers, with 480 nm being a common average value. In contrast, red light has a longer wavelength, generally falling between 620 and 750 nanometers, and 650 nm is a standard value often used to represent it.
MULTIPLE CHOICE TYPE:
Question 1:
When a white light ray falls on a prism, the ray at its first surface suffers.
(a) no refraction
(b) only dispersion
(c) only deviation
(d) both deviation and dispersion
Ans: (d) both deviation and dispersion
Question 2:
In the spectrum of white light by a prism, the colour at the extreme end opposite to the base of prism is:
(a) violet
(b) yellow
(c) red
(d) blue
Ans:
(c) red
NUMERICALS:
Question 1:
Calculate the frequency of yellow light of wavelength 550nm. The speed of light is 3×108 m/s
Ans: Step 1: Write down the known values
Wavelength,
λ=550 nm=550×10 −9 m
Speed of light,
c=3×10 8 m/s
Step 2: Use the wave equation
c=fλ
f=c/λ
Step 3: Substitute values
f=3×10*8/550×10−93×10 *8
Step 4: Calculate
f=3/5.5×10*15
f≈0.545×10 *15 Hz
Final Answer:5.45×10 *14 Hz
Question 2:
The frequency range of visible light is from 3.75×1014 Hz to 7.5×1014 Hz. Calculate its wavelength range. Take speed of light =3×108 m/s
Ans: Step 1: Understanding the relationship
We know the relationship between the speed of light (c), frequency (f), and wavelength (λ) is:
c=fλ
Rearranging for wavelength:
λ=c/f
Where:
c=3×10 *8m/s
Step 2: Wavelength for the lower frequency
Lower frequency
f =3.75×10 *14 Hz
λ1=3×10*8
= 3.75×10 *14
m=800nm
Step 3: Wavelength for the higher frequency
Higher frequency
f2 =7.5×10 *14 Hz
m=400nm
Step 4: Final wavelength range
The wavelength range is from 400 nm to 800 nm.
400 nm to 800 nm
EXERCISE 6(B)
Question 1:
(a) Give a list of at least five radiations, in order of their increasing frequencies, which make up the complete electromagnetic spectrum.
(b) Which of the radiation mentioned in answer to part (a) has the highest penetrating power?
Ans: (a) List of five radiations in order of increasing frequency:
Radio waves
Microwaves
Infrared radiation
Visible light
Ultraviolet radiation
(b) Radiation with the highest penetrating power:
Gamma rays (not listed above, but they have the highest penetrating power in the complete electromagnetic spectrum).
From the list in part (a), ultraviolet radiation has the highest penetrating power.
Question 2:
(a) Arrange the following radiations in the order of their increasing wavelength: X-rays, infrared rays, radio waves, gamma ray and micro waves.
(b) Which radiation is used for satellite communication?
Ans: (a) Order of increasing wavelength:
Gamma rays < X-rays < Infrared rays < Microwaves < Radio waves
(b) Radiation used for satellite communication:
Microwaves
Explanation:
Gamma rays have the shortest wavelength.
Radio waves have the longest wavelength.
Microwaves are used for satellite communication because they can penetrate through the Earth’s atmosphere effectively.
Question 3:
A wave has a wavelength of 0.01 Å.
(a) Name the wave.
(b) State its one property different from light.
Ans:
(a) Name the wave.
The wave is a Gamma ray.
(b) State its one property different from light.
One key property of gamma rays that is different from visible light is their much higher penetrating power. While visible light can be blocked by opaque objects like paper or wood, gamma rays can pass easily through several centimeters of lead or concrete.
Question 4:
(a) Name the high energetic invisible electro-magnetic wave which helps in the study of structure of crystals.
(b) State one more use of the wave named in part (a).
Ans: (a) X-rays
X-rays are a type of high-energy electromagnetic radiation. They have a very short wavelength, which is even shorter than the wavelength of ultraviolet light but longer than gamma rays. Due to their high energy, X-rays can pass through many materials that visible light cannot, such as soft tissues in the human body. However, they are partially absorbed by denser materials like bones or metals. This property makes them extremely useful in various fields, especially in medicine and security.
(b) They are used in medical imaging to examine broken bones or dental problems.
In medicine, X-rays are primarily used for diagnostic imaging. When a part of the body is exposed to X-rays, the denser structures (such as bones) absorb more radiation and appear white on the special film or digital detector. Softer tissues, which allow more X-rays to pass through, appear in shades of gray. This creates a clear image, known as a radiograph, which allows doctors to see inside the body without surgery. This is crucial for quickly and accurately diagnosing fractures, checking for tooth decay and other dental issues, locating foreign objects, and screening for conditions like pneumonia or certain types of cancer.
Question 5:
What is the range of the wavelength of the following electromagnetic waves?
(a) Gamma rays
(b) X-rays
(c) Ultraviolet
(d) Visible
(e) Infrared
(f) Micro waves
(g) Radio waves
Ans: (a) Gamma rays Wavelength range: Less than 0.01 nanometres (nm)
(b) X-rays Wavelength range: 0.01 nm to 10 nm
(c) Ultraviolet rays Wavelength range: 10 nm to 400 nm
(d) Visible light Wavelength range: 400 nm to 700 nm
(e) Infrared rays Wavelength
(f) Microwaves Wavelength range: 1 mm to 30 centimetres (cm)
(g) Radio waves Wavelength range: 30 cm and above (can be several kilometres long)
Question 6:
Give the range of wavelength of the electromagnetic waves visible to us.
Ans: The range of wavelengths of electromagnetic waves that are visible to the human eye is from approximately 400 nanometres (nm) to 700 nanometres (nm). This band of light is what we commonly perceive as the colours of the rainbow, with violet and blue at the shorter end (around 400 nm) and red at the longer end (around 700 nm).
Question 7:
Name the region beyond (i) the red end and (ii) the violet end, of the spectrum.
Ans: The region of the electromagnetic spectrum that lies beyond the visible light spectrum has specific names:
(i) The region beyond the red end is known as the infrared region. We cannot see this radiation, but we can often feel it as heat.
(ii) The region beyond the violet end is known as the ultraviolet region. This light is also invisible to our eyes but is known for its effects, like causing sunburn and helping our bodies produce Vitamin D.
Question 8:
What do you understand by the invisible spectrum? How will you investigate the existence of radiation beyond the red and violet ends of the spectrum?
Ans: The Invisible Spectrum
The invisible spectrum refers to the bands of electromagnetic radiation that lie just beyond the red and violet ends of the visible light spectrum. While our eyes are sensitive to the range of colours from violet to red, they cannot detect these adjacent regions. The part beyond the red end is called the infrared region, and the part beyond the violet end is called the ultraviolet region. Even though we cannot see them, these radiations exhibit specific effects that prove their existence.
Investigating Radiation Beyond the Red End (Infrared Radiation)
To investigate the existence of infrared radiation, a simple experiment can be performed using a thermopile and a sensitive galvanometer.
A spectrum is projected onto a screen using a glass prism.
A thermopile (a device that converts thermal energy into electrical energy) is placed just beyond the red end of the visible spectrum, where no visible light falls.
The thermopile is connected to a sensitive galvanometer.
It is observed that the galvanometer shows a deflection, indicating that an electric current is being generated.
This current is produced because the thermopile is being heated by some form of energy falling on it. This energy is the invisible infrared radiation.
Investigating Radiation Beyond the Violet End (Ultraviolet Radiation)
The existence of ultraviolet radiation can be demonstrated by its ability to cause fluorescence in certain materials.
A spectrum is projected onto a screen, but this time, the screen is coated with a material that fluoresces, such as a solution of quinine sulphate or a piece of white cloth stained with a fluorescent dye.
When the setup is observed in a darkened room, a bright, glowing patch of light becomes visible extending beyond the violet end of the spectrum.
This glow is not the ultraviolet light itself, but rather visible light emitted by the fluorescent material when it absorbs the invisible, high-energy ultraviolet radiation.
These experiments confirm that the visible spectrum is only a small part of a much broader electromagnetic spectrum, with active but invisible radiations bordering it on both sides.
Question 9:
State the approximate range of wavelength associated with (i) the ultraviolet rays, (ii) the visible light and (iii) infrared rays.
Ans: The electromagnetic spectrum is divided into different regions based on wavelength. The approximate wavelength ranges for the mentioned rays are:
(i) Ultraviolet Rays:
Ultraviolet (UV) rays have shorter wavelengths than visible light, which makes them invisible to the human eye. They are known for their effects like causing sunburn and helping our bodies produce Vitamin D. Their wavelength typically ranges from about 100 Å to 4000 Å (or, equivalently, 10 nm to 400 nm).
(ii) Visible Light:
This is the very narrow band of the spectrum that our eyes can detect. Within this range, different wavelengths are perceived as different colours, from violet at the short end to red at the long end. The approximate wavelength range for visible light is from 4000 Å to 8000 Å (or 400 nm to 800 nm).
(iii) Infrared Rays:
Infrared (IR) rays have longer wavelengths than visible red light. We cannot see them, but we can feel them as heat. For example, the warmth from a radiator or the sun is largely due to infrared radiation. Their wavelength typically ranges from 8000 Å to 10⁷ Å (or 800 nm to 1 mm).
Question 10:
Name the radiations of wavelength just (i) longer than 8×10−7 (ii) shorter than 4×10−7 m.
Ans:
beyond this, for wavelengths longer than 8×10⁻⁷ m, we find Infrared Radiations. These are often felt as heat.
(ii) The wavelength 4 × 10⁻⁷ m (or 400 nm) is the other end of the visible spectrum. For wavelengths shorter than 4×10⁻⁷ m, we encounter Ultraviolet Radiations. These are the rays from the sun that can cause sunburn.
Question 11:
Name two electromagnetic waves of frequency greater than that of violet light. State one use of each.
Ans: Two types of electromagnetic waves that have frequencies even higher than violet light are Ultraviolet (UV) Rays and X-Rays.
1. Ultraviolet (UV) Rays
These waves are invisible to our eyes but carry more energy than violet light. A very common and important use for them is in water purification systems. In these devices, UV light is shone through water, and its high energy damages the DNA of harmful bacteria, viruses, and other microbes. This process effectively kills or sterilizes them, making the water safe for drinking without the need for chemicals like chlorine.
2. X-Rays
X-rays possess even more energy and penetrating power than UV rays. Their most well-known application is in the field of medical imaging. Because X-rays can pass through soft tissues like skin and muscle but are largely blocked by denser materials like bones and metals, they are perfect for creating images of the inside of the body. Doctors rely on X-ray images to diagnose and examine fractures in bones, check for dental cavities, and locate other internal issues.
Question 12:
Give one use each of (i) microwaves, (ii) ultraviolet radiations, (iii) infrared radiations, and (iv) gamma rays.
Ans: (i) Microwaves: You’ll find these hard at work in your kitchen’s microwave oven. They heat food by making the water molecules inside it vibrate really fast, which creates the heat that warms or cooks your meal in minutes.
(ii) Ultraviolet Radiations: These are the secret weapon in many water purifiers. They work by zapping harmful bacteria and viruses, damaging their genetic material so they can’t multiply, which leaves the water clean and safe for drinking.
(iii) Infrared Radiations: This is the technology behind your TV remote. When you press a button, the remote sends out an invisible infrared signal that travels to the TV, telling it to change the channel or turn up the volume.
(iv) Gamma Rays: In the medical field, these powerful rays are used in cancer treatment. During radiotherapy, doctors carefully focus gamma rays on a tumour. The intense energy from the rays helps to kill the cancer cells and stop them from growing.
Question 13:
Name the rays or waves
(i) of highest frequency,
(ii) used for taking photographs in dark,
(iii) produced by the changes in the nucleus of an atom,
(iv) of wavelength nearly 0.1 nm.
Ans:(i) Gamma rays have the highest frequency in the electromagnetic spectrum. They carry the most energy and are produced by the most powerful and violent events in the universe, like nuclear reactions or cosmic phenomena.
(ii) Infrared rays are used for taking photographs in the dark. This works because every object gives off some amount of heat, which is a form of infrared radiation. Special cameras or night-vision goggles can detect this invisible radiation and convert it into a visible image, allowing us to “see” in the dark.
(iii) Gamma rays are produced by changes in the nucleus of an atom. This typically happens during radioactive decay, when an unstable atomic nucleus releases energy to become more stable, often emitting a gamma ray in the process.
(iv) X-rays have a wavelength of nearly 0.1 nm. This very short wavelength allows them to pass through soft tissues in our body (like skin and muscle) but are mostly absorbed by denser materials like bones or metals. This property is precisely why they are so useful in medical imaging to check for fractures and in security scanners to see inside luggage.
Question 14:
Two waves A and B have wavelength 0.01 Å and 9000 Å respectively.
(a) Name the two waves.
(b) Compare the speeds of these waves when they travel in vacuum.
Ans: (a) Name the two waves.
Wave A (0.01 Å): This is an extremely short-wavelength wave, characteristic of Gamma rays. Gamma rays are produced from nuclear reactions and are a form of high-energy ionizing radiation.
Wave B (9000 Å): This wavelength falls within the infrared region of the electromagnetic spectrum. Specifically, 9000 Å (or 900 nanometers) is in the Near-Infrared range.
(b) Compare the speeds of these waves when they travel in vacuum.
Both waves, despite being very different in wavelength and nature, travel at the same speed in a vacuum.
This is because all electromagnetic waves, from long radio waves to short gamma rays, propagate through a vacuum at the universal constant speed of light, which is approximately 3 × 10⁸ m/s. The speed is independent of their wavelength or frequency.
Question 15:
Name two sources, each of infrared radiations and ultraviolet radiations.
Ans: Infrared Radiations:
The Sun: The sun is a primary natural source of infrared radiation. A significant portion of the solar energy that reaches the Earth is in the form of infrared rays, which we feel as heat.
A Hot Electric Iron or a Heater: Common household appliances that get very hot, like an electric room heater or the soleplate of an iron, are everyday examples of man-made sources that emit a substantial amount of infrared radiation.
Ultraviolet Radiations:
The Sun: Similar to infrared, the sun is also a major natural source of ultraviolet (UV) radiation. The UV rays from the sun are responsible for causing sunburns and also help in the natural production of Vitamin D in our bodies.
Mercury Vapour Lamps: Certain specialized electric lamps, such as those used in mercury vapour lamps or the “black lights” seen in discos, are designed to produce ultraviolet light. They work by passing an electric current through vaporized mercury.
Question 16:
What are infrared radiations? How are they detected? State one use of these radiations.
Ans: Infrared radiation is a type of invisible light that we feel as heat. Any warm object, from the sun to a cup of coffee, gives off this energy. Our skin can sense it, but tools like thermopiles are used to measure it accurately by turning the heat into an electrical signal.
A very common everyday use is in a TV remote control. When you press a button, the remote flashes a coded pattern of infrared light. A receiver on the television picks up this pattern and carries out your command, like changing the channel.
Question 17:
What are ultraviolet radiations? How are they detected? State one use of these radiations.
Ans: Ultraviolet Radiations:
Ultraviolet (UV) radiations are electromagnetic waves with wavelengths shorter than visible light but longer than X-rays.
Detection:
UV radiations are detected using fluorescent materials, which glow when exposed to them. For example, zinc sulphide or certain dyes fluoresce under UV light. Photographic films and specialised UV sensors can also detect them.
One Use:
UV radiations are used in water purification to kill harmful bacteria and viruses, making water safe to drink.
Question 18:
Name three properties of ultraviolet radiations which are similar to visible light.
Ans: Ultraviolet radiations share the following three properties with visible light:
Travel in Straight Lines: Like visible light, ultraviolet rays travel in straight lines in a homogeneous medium.
Obey Laws of Reflection and Refraction: Ultraviolet radiations follow the laws of reflection (angle of incidence equals angle of reflection) and refraction (change in direction when passing from one medium to another).
Electromagnetic Wave Nature: Both ultraviolet and visible light are part of the electromagnetic spectrum and exhibit wave properties such as interference, diffraction, and polarization.
Question 19:
Give two properties of ultraviolet radiations which differ from the visible light.
Ans: The two properties of ultraviolet radiations which differ from visible light are:
Higher Energy and Photochemical Effects: Ultraviolet (UV) radiations possess significantly higher energy compared to visible light. This higher energy allows UV rays to initiate chemical reactions that visible light cannot. A common example is the way UV radiation causes our skin to produce vitamin D and leads to tanning or sunburn, whereas visible light does not have this effect. This property is also utilized in processes like disinfecting water and surfaces, where UV light breaks down the DNA of bacteria and viruses.
Invisibility and Interaction with Materials: Unlike visible light, which our eyes are designed to detect, ultraviolet radiation is invisible to the human eye. Furthermore, UV light interacts with certain materials in a unique way through a phenomenon called fluorescence. Many substances, like the dyes in white clothes or some minerals, can absorb invisible ultraviolet radiation and then re-emit the energy as visible light, making them appear to glow. Visible light does not produce this dramatic fluorescent effect.
Question 20:
Mention three properties of infrared radiations similar to the visible light.
Ans: Infrared radiations share the following three properties with visible light:
Travel in Straight Lines: Like visible light, infrared rays propagate in straight lines in a homogeneous medium.
Follow Laws of Reflection: They obey the laws of reflection, where the angle of incidence equals the angle of reflection.
Can Be Refracted: Infrared radiations undergo refraction when passing from one medium to another, changing direction at the interface.
Question 21:
Give two such properties of infrared radiations which are not true for visible light.
Ans: Infrared radiation has some key differences from visible light. Two major properties are:
Sensing Heat: Our skin detects infrared radiation primarily as heat. For example, we can feel the warmth from a fireplace or the sun on our skin even with our eyes closed because we are sensing its infrared rays. Visible light, on the other hand, does not produce this same sensation of warmth; we see it, but we don’t primarily “feel” it as heat.
Penetrating Ability: Infrared rays are better at passing through certain things that visible light cannot. For instance, in foggy or dusty conditions, visible light gets scattered easily, making it hard to see. However, infrared radiation can penetrate haze and mist more effectively, which is why infrared cameras and sensors are used for visibility in such environments.
Question 22:
Explain the following:
(i) Infrared radiations are used for photography in fog.
(ii) Infrared radiations are used as signals during war.
(iii) The photographic darkrooms are provided with infrared lamps.
(iv) A rock salt prism is used instead of a glass prism to obtain the infrared spectrum.
(v) A quartz prism is required for obtaining the spectrum of the ultraviolet light.
(vi) Ultraviolet bulbs have a quartz envelope instead of glass.
Ans: (i) Infrared radiations are used for photography in fog.
Fog, rain, or haze scatter visible light significantly, which is why it’s difficult to see or photograph distant objects in such conditions. However, infrared radiations have longer wavelengths compared to visible light. These longer wavelengths are less scattered by the tiny water droplets and particles in the fog. Because they can penetrate the fog more effectively, infrared photography allows for clearer images to be captured in poor weather conditions that would otherwise obscure the view.
(ii) Infrared radiations are used as signals during war.
During military operations, secure and covert communication is vital. Infrared signals are invisible to the naked human eye, making them ideal for sending messages between troops, vehicles, or bases without revealing their position to an enemy who is not equipped with infrared detection devices. This allows forces to coordinate movements and strategies secretly.
(iii) The photographic darkrooms are provided with infrared lamps.
A photographic darkroom is used to process light-sensitive film and paper, which must be done in complete darkness or under a specific safe light to avoid exposure. Normal visible light would ruin the material. Infrared lamps are used because photographic films and papers are generally not sensitive to the longer wavelengths of infrared light. This provides illumination for the person working, allowing them to see what they are doing without fogging or exposing the light-sensitive materials.
(iv) A rock salt prism is used instead of a glass prism to obtain the infrared spectrum.
Glass is opaque to much of the infrared region of the electromagnetic spectrum; it absorbs infrared radiation rather than transmitting it. To study the infrared spectrum, the prism must be made of a material that allows these wavelengths to pass through. Rock salt (like sodium chloride) is transparent to a wide range of infrared radiations, making it a suitable material for constructing prisms and lenses for infrared spectroscopes.
(v) A quartz prism is required for obtaining the spectrum of the ultraviolet light.
Just as glass blocks infrared, ordinary glass absorbs ultraviolet radiations. To study the ultraviolet spectrum, the optical components must be made from a material that is transparent to UV light. Quartz (or fused silica) is highly transparent to ultraviolet radiation, allowing it to be used effectively in prisms and lenses for spectroscopes designed to analyze UV light.
(vi) Ultraviolet bulbs have a quartz envelope instead of glass.
Ultraviolet lamps are designed to emit UV radiation. If they were made with ordinary glass, the glass envelope would absorb most of the beneficial ultraviolet rays, preventing them from escaping the bulb. Quartz, being transparent to a broad range of UV wavelengths, is used as the envelope material so that the ultraviolet light produced inside the bulb can be transmitted outwards effectively for its intended use, such as in sterilization, tanning beds, or scientific application
MULTIPLE CHOICE TYPE:
Question 1:
The most energetic electromagnetic radiations are:
(a) Microwaves
(b) ultraviolet waves
(c) X-rays
(d) gamma rays
Ans: (d) gamma rays
Question 2:
The source of ultraviolet light is:
(a) electric bulb
(b) red hot iron ball
(c) sodium vapour lamp
(d) carbon arc-lamp
Ans: (d) carbon arc-lamp
Question 3:
A radiations A is focused by a proper device on the bulb of a thermometer. Mercury in the thermometer shows a rapid increase. The radiations A is:
(a) infrared radiation
(b) visible light
(c) ultraviolet radiation
(d) X-rays
Ans: (b) visible light
NUMERICALS
Question 1:
An electromagnetic wave has a frequency of 500 MHz and a wavelength of 60 cm.
(a) Calculate the velocity of the wave.
(b) Name the medium through which it is travelling.
Ans: (a) Calculate the velocity of the wave.
We know the relationship between the velocity (v), frequency (f), and wavelength (λ) of a wave is:
v = f × λ
Given:
Frequency, f = 500 MHz = 500 × 10⁶ Hz = 500,000,000 Hz
Wavelength, λ = 60 cm = 0.6 m
Now, let’s substitute the values into the formula:
v = (500 × 10⁶ Hz) × (0.6 m)
v = 500,000,000 × 0.6
v = 300,000,000 m/s
∴ The velocity of the wave is 3 × 10⁸ m/s.
(b) Name the medium through which it is travelling.
The calculated velocity of 3 × 10⁸ m/s is the speed of light in a vacuum. Since electromagnetic waves do not require a material medium to travel and this wave is moving at the speed of light, it is most likely travelling through air or vacuum.
Air has a refractive index very close to 1, so the speed of light in air is almost identical to its speed in a vacuum. Therefore, the medium is most simply identified as:
Vacuum (or Air).
EXERCISE 6(C)
Question 1:
What is meant by scattering of light?
Ans: Scattering of light is what happens when tiny particles or molecules deflect light from its straight path, sending it in various new directions. Think of it as light being bounced around by small obstacles.
The most common example is our blue sky. Sunlight seems white, but it actually contains all colors. The gases in our atmosphere scatter blue light more easily than other colors like red. So, when we look up, we see this scattered blue light coming from all over, which makes the entire sky appear blue to our eyes.
Question 2:
How does the intensity of scattered light depend on the wavelength of incident light? State conditions when this dependence holds.
Ans:
When light strikes small particles, the intensity of the scattered light is inversely proportional to the fourth power of the wavelength of the incident light. This relationship is known as Rayleigh’s Law.
Mathematically, this is expressed as:
I ∝ 1 / λ⁴
This means that shorter wavelengths (like blue and violet light) are scattered much more strongly than longer wavelengths (like red and orange light).
Conditions for this Dependence:
This relationship holds under the following conditions:
The size of the scattering particles must be much smaller than the wavelength of the incident light (e.g., gas molecules like nitrogen and oxygen in the atmosphere).
The scattering medium is a clear, transparent substance.
Question 3:
When sunlight enters the earth’s atmosphere, state which colour of light is scattered the most and which the least.
Ans: When sunlight travels through space and reaches Earth, it looks white to us. But this white light is actually a mix of all the colours of the rainbow, each with its own wavelength.
As this sunlight enters our atmosphere, it collides with the tiny molecules of gases and other particles in the air. This collision causes the light to scatter in different directions. Now, not all colours scatter equally. The shorter wavelengths of light, like violet and blue, are much more easily scattered by these tiny molecules because they interact with them more strongly. Think of it like the smaller, quicker blue and violet waves getting bounced around more easily than the longer, more steady waves.
Interestingly, the colour that gets scattered the absolute most is actually violet. So, why doesn’t our sky look violet? It’s because of our eyes. The sensors in our human eyes are simply more sensitive to blue light and less sensitive to violet. Therefore, even though violet is being scattered slightly more, our brains perceive the brilliant blue as the dominant colour across the sky.
On the other hand, the colour with the longest wavelength, red, is scattered the least. It tends to push through the atmosphere without getting bounced around much. This is the secret behind the breathtaking red and orange sunsets we love. During sunrise and sunset, the sun is low on the horizon, and its light has to travel through a much thicker portion of the atmosphere to reach our eyes. All that extra air scatters away most of the blue and violet light, leaving the stubborn, unscattered reds, oranges, and yellows to travel straight to our eyes, painting the sky with their warm glow.
Question 4:
The danger signal is red. Why?
Ans: The danger signal is red because red light has the longest wavelength in the visible spectrum. This allows it to be scattered the least by air molecules and other particles, enabling it to travel the farthest through fog, smoke, or rain and remain clearly visible from a long distance.
Question 5:
How would the sky appear when seen from the space (or moon)? Give reason for your answer.
Ans: When seen from space or the moon, the sky appears black instead of blue.
Reason: On Earth, the sky appears blue because our atmosphere scatters sunlight, especially the blue light. In space or on the moon, there is no atmosphere to scatter the sunlight. Therefore, sunlight travels in straight lines without scattering, and no light reaches our eyes from the sky’s direction, making it look dark or black. We see the stars clearly even during the day for the same reason.
Question 6:
What characteristic property of light is responsible for the blue colour of the sky?
Ans: Scattering of light.
Sunlight contains different colours, and the Earth’s atmosphere has many tiny molecules and dust particles. These particles scatter the shorter wavelengths of light (blue and violet) more effectively than the longer wavelengths (red and orange).Since our eyes are more sensitive to blue light than violet, we see the sky as blue.
Question 7:
The colour of sky, in direction other than of the sun, is blue. Explain.
Ans: The sky appears blue in directions away from the sun due to a phenomenon called Rayleigh scattering.
Sunlight is made up of different colours (wavelengths). When it enters Earth’s atmosphere, it collides with gas molecules and tiny dust particles.
Shorter wavelengths of light (like blue and violet) are scattered much more strongly than longer wavelengths (like red and orange). Since our eyes are more sensitive to blue light than violet, the sky appears blue from every direction except towards the sun itself. This scattered blue light reaches our eyes from all parts of the sky, making it look blue.
Question 8:
Why does the sun appear red at sunrise and sunset?
Ans:
At sunrise and sunset, the sun is near the horizon.
The atmosphere scatters shorter wavelengths of light (like blue and violet) away from our line of sight. However, the longer wavelengths, like red and orange, are scattered much less and pass straight through.
This is why mostly the red light reaches our eyes directly, making the sun appear red.
Question 9:
The sky at noon appears white. Give reason.
Ans: The sky at noon appears white because at that time, sunlight travels through the least amount of atmosphere. This causes shorter-wavelength blue light to scatter only slightly, while other colours like red and yellow also scatter a little. All these scattered colours mix together, making the sky appear white to our eyes.
Question 10:
The clouds are seen white. Explain.
Ans: Sunlight might look plain and white to us, but it’s actually made up of seven different colours mixed together. You can see them separated in a rainbow. Now, the water droplets that make up a cloud are relatively large. Because of their size, they don’t play favourites with any particular colour. When sunlight hits these droplets, they scatter all seven colours equally.
This means that red, orange, yellow, green, blue, indigo, and violet light are all sent bouncing around inside the cloud in the same amounts. When our eyes receive this balanced mixture of every colour, our brain simply interprets it as white light.
MULTIPLE CHOICE TYPE:
Question 1:
In white light of sun, maximum scattering by the air molecular present in the earth’s atmosphere is for:
(a) red colour
(b) yellow colour
(c) green colour
(d) blue colour Ans: (d) blue colour


