The foundation of understanding biological processes lies in grasping the language of chemistry, which begins with elements and atoms. An element is a pure substance made of only one kind of atom, and it is the fundamental building block of all matter, whether living or non-living. These atoms consist of a central nucleus, containing protons and neutrons, surrounded by electrons in specific orbits. The key to an atom’s chemical behavior is its atomic structure, particularly the number of electrons in its outermost shell, which determines how it will bond with other atoms to achieve stability. This quest for stability drives the formation of molecules and compounds, which are essential for life itself.
Atoms interact through chemical bonding, primarily by sharing or transferring electrons. When atoms share electrons, a covalent bond is formed, creating molecules like oxygen (O₂) or water (H₂O), which are crucial for respiration and as a biological solvent. In contrast, ionic bonds form when electrons are completely transferred from one atom to another, resulting in the creation of positively and negatively charged ions that attract each other. Furthermore, the chapter explains how these combinations of elements are represented through chemical formulas, which use symbols and subscripts to denote the type and number of atoms present in a single molecule of a substance.
To communicate the dynamic changes in matter, chemists use chemical equations. These equations are symbolic representations where the reactants (the starting substances) are shown on the left, and the products (the substances formed) are on the right, separated by an arrow. A critical principle in chemistry is the Law of Conservation of Mass, which states that matter cannot be created or destroyed. Therefore, a chemical equation must be “balanced,” meaning the number of atoms for each element is the same on both sides. This balancing is achieved by placing numbers, called coefficients, in front of the formulas, ensuring the equation accurately reflects the chemical transformation.
Exercise 1 (A)
Question 1.
What is a symbol? What information does it convey?
Ans:
| Type of Information | Description | Example |
| Identity and Name | The most basic function; it acts as a unique, concise abbreviation for a specific entity. | The chemical symbol O identifies the element Oxygen. |
| Representation | It represents a complex concept or idea in a simplified, recognizable form. | A red octagon traffic sign symbolizes the complex instruction: “STOP.” |
| Quantitative Data | In chemistry and mathematics, the symbol represents a definite quantity or value. | The symbol O represents one atom of Oxygen and one gram atomic mass of Oxygen. |
| Emotional/Abstract Meaning | Symbols evoke specific feelings, beliefs, or abstract concepts that are learned culturally. | A red rose often symbolizes love and passion. |
| Cultural/Ideological Value | They serve as unifying emblems for groups, conveying shared values, traditions, or social structures. | A national flag is a symbol of a country’s sovereignty and identity. |
Question 2.
Why is the symbol S for sulphur, but Na for sodium and Si for silicon?
Ans:
The seemingly inconsistent use of symbols for elements like Sulfur, Sodium, and Silicon is due to the convention that the International Union of Pure and Applied Chemistry (IUPAC) uses to derive them.
The symbols are derived from either the English name of the element or, more commonly for older elements, their Latin or Greek name.
Symbol Derivation
1. S for Sulfur and Si for Silicon (From English Name)
For these elements, the symbol is derived directly from their modern English names.
- S for Sulfur: Uses the first letter of the English name.
- Si for Silicon: Uses the first two letters of the English name (since S was already taken by Sulfur).
2. Na for Sodium (From Latin Name)
For some of the oldest known elements, the symbol is derived from their historical Latin name instead of the modern English name.
- Na for Sodium: The symbol comes from its Latin name, Natrium.
- Natrium is derived from natron, a naturally occurring mineral salt (sodium carbonate) used historically.
Summary of Rules
The IUPAC convention uses three main rules for element symbols:
- Use the first letter of the English name (S for Sulfur).
- If the first letter is taken, use the first two distinct letters of the English name (Si for Silicon, He for Helium).
- For historically important elements, use the first one or two letters of their Latin name (Na for Natrium, Fe for Ferrum (Iron), Au for Aurum (Gold)).
Question 3.
Write the full form of IUPAC. Name the elements represented by the following symbols:
Au, Pb, Sn, Hg
Ans:
The full form of IUPAC is the International Union of Pure and Applied Chemistry.
It is the world authority that sets the standard nomenclature and terminology for chemical science, including the naming of elements and chemical compounds.
Elements Represented by Symbols
The elements represented by the given symbols, which are derived from their historical Latin names, are:
| Symbol | Element Name | Latin Name (Origin) |
| Au | Gold | Aurum |
| Pb | Lead | Plumbum |
| Sn | Tin | Stannum |
| Hg | Mercury | Hydrargyrum |
Question 4.
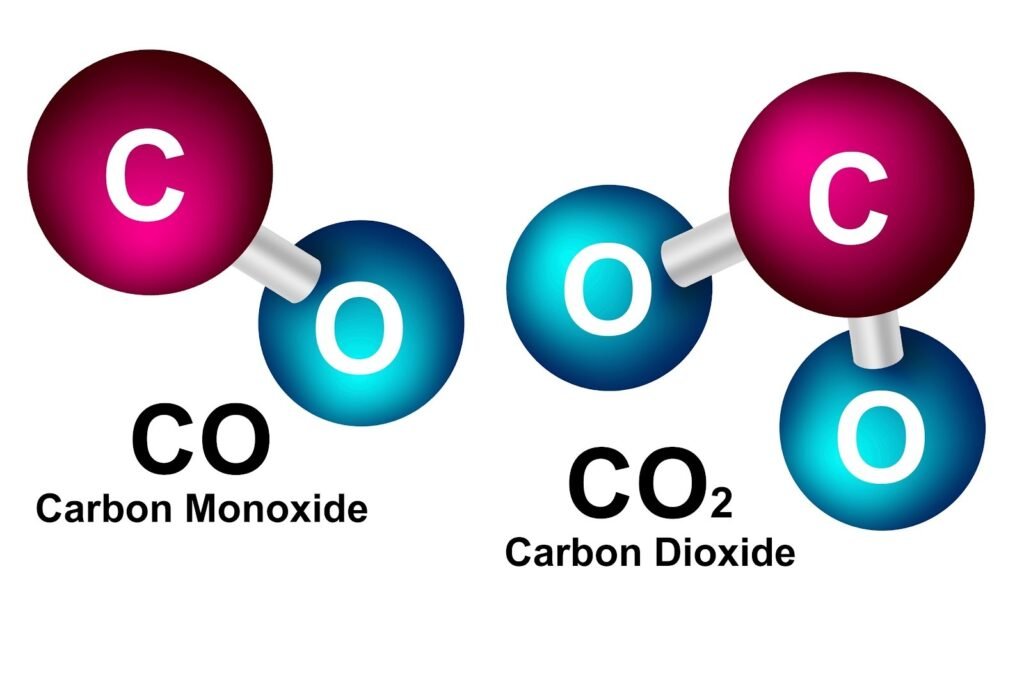
If the symbol for Cobalt, Co, were written as CO, what would be wrong with it?
Ans:
If the symbol for the element Cobalt were written as CO instead of Co, it would be confused with the chemical formula for Carbon Monoxide.
This illustrates the crucial rules of chemical nomenclature set by IUPAC:
- Element Symbol: The symbol for a single element is always written with the first letter capitalized and the second letter (if present) in lowercase (e.g., Co for Cobalt).
- Compound Formula: A chemical formula consisting of two capitalized letters indicates a compound made up of two different elements (e.g., CO for Carbon Monoxide, which is one atom of Carbon (C) and one atom of Oxygen (O))
Therefore, writing CO would incorrectly represent a molecule of a compound rather than a single atom of the element Cobalt.
Question 5.
1. What do the following symbols stand for? H
2. What do the following symbols stand for? H2
3. What do the following symbols stand for? 2H
4. What do the following symbols stand for? 2H2
Ans:
In chemical notation, the distinction between symbols, subscripts, and coefficients is fundamental. The symbol H represents a single, isolated atom of the element hydrogen, the basic unit. When we write H₂, the small subscript ‘2’ indicates a molecule where two hydrogen atoms are chemically bonded together; this is the natural, stable form of hydrogen gas. It represents a single, distinct unit of this gaseous substance.
The numbers placed in front of these symbols, known as coefficients, tell us the quantity. Therefore, 2H specifies two separate and unconnected hydrogen atoms, which is a rare condition as atoms tend to form bonds. Finally, 2H₂ describes two distinct molecules of hydrogen gas. Since each molecule contains two bonded atoms, this notation represents a total of four hydrogen atoms, grouped into two stable, paired sets. Understanding this notation is key to reading the language of chemistry correctly.
Question 6.
What is meant by atomicity? Name the diatomic element.
Ans:
Atomicity refers to the total number of atoms that are chemically bonded together to form a single molecule of an element. In simpler terms, it tells us how many atoms are present in one molecule of that specific element. This property helps classify elements based on the structure of their basic molecular unit in nature. For example, some elements are stable as single atoms, while others naturally pair up or form larger groups.
Elements that exist as molecules made of two atoms are called diatomic elements. A classic and biologically crucial example of a diatomic element is Oxygen, whose molecule is represented as O₂. Besides oxygen, several other important gases are also diatomic, including Nitrogen (N₂), Hydrogen (H₂), Chlorine (Cl₂), Fluorine (F₂), Bromine (Br₂), and Iodine (I₂). These seven elements are generally found as stable pairs of atoms in their natural state.
Question 7.
1. Explain the terms ‘valency’ and ‘variable valency’.
2. How are the elements with variable valency named? Explain with an example.
Ans:
1. Valency and Variable Valency
Valency
Valency is the combining capacity of an element. It is defined by the number of chemical bonds that an atom of an element can form with other atoms.
- In simple terms, valency is the number of electrons an atom must gain, lose, or share to achieve a stable electronic configuration (like a noble gas), typically having eight electrons in its outermost shell (the octet rule).
- For example, the valency of Oxygen (O) is 2 because it needs to gain two electrons to complete its octet.
Variable Valency
Variable valency is the property exhibited by certain elements, particularly transition metals, to show more than one stable valency or combining capacity in their compounds.
- This means an atom of such an element can react by sharing or transferring a different number of electrons depending on the specific reaction conditions or the element it is bonding with.
- For example, the element Iron (Fe) can have a valency of both 2 and 3.
2. Naming Elements with Variable Valency nomenclature
Elements that exhibit variable valency are named using one of two standard nomenclature systems: the Older (Classical) System or the Stock System (IUPAC).
A. Older (Classical) System
This system uses Latin roots and suffixes to distinguish between the two valencies:
- Suffix -ous: Used for the compound where the element has the lower valency.
- Suffix -ic: Used for the compound where the element has the higher valency.
| Valency | Latin Root | Suffix | Name |
| Lower (+2) | Ferr | -ous | Ferrous (e.g., FeCl2: Ferrous Chloride) |
| Higher (+3) | Ferr | -ic | Ferric (e.g., FeCl3: Ferric Chloride) |
B. Stock System (IUPAC System)
This modern system is more systematic and precise. It uses Roman numerals in parentheses immediately following the English name of the metal to directly indicate its valency (or oxidation state) in that particular compound.
| Valency | Example Compound | IUPAC Name |
| Lower (2) | FeCl2 | Iron (II) Chloride |
| Higher (3) | FeCl3 | Iron (III) Chloride |
Question 8.
1.Give the formula and valency of:
| Aluminate | ______ | ______ |
2. Give the formula and valency of: chromate ………….…….. .
3. Give the formula and valency of: aluminium ………………. .
4. Give the formula and valency of: cupric ………………… .
Ans:
1. Aluminate
- Formula: AlO₂⁻
- Valency: -1
The aluminate ion is an oxyanion where aluminium is the central atom. Its valency of -1 indicates its tendency to gain one electron or form one bond to achieve stability.
2. Chromate
- Formula: CrO₄²⁻
- Valency: -2
The chromate ion carries two units of negative charge. This -2 valency means it can combine with two singly charged positive ions or one doubly charged positive ion.
3. Aluminium
- Formula: Al
- Valency: +3
As an element, aluminium atom tends to lose its three outermost electrons to form the Al³⁺ cation. This gives it a stable valency of +3 in its compounds.
4. Cupric
- Formula: Cu²⁺
- Valency: +2
The term “cupric” refers to the copper(I) ion. It has a valency of +2, showing that it has a tendency to form compounds by donating two electrons.
Question 9.
What is a chemical formula? What is the significance of a formula?give an example to illustrate?
Ans:
A chemical formula is a symbolic representation that uses element symbols and numerical subscripts to convey the precise type and number of atoms present in a single molecule of a substance. It serves as a fundamental shorthand in chemistry, providing a wealth of information in a compact form. For instance, the formula H₂O immediately tells us that one molecule of this substance is composed of two hydrogen (H) atoms and one oxygen (O) atom.
The significance of a chemical formula is multi-layered. Primarily, it identifies the specific elements that constitute a compound. Beyond mere identification, it reveals the exact atomic composition, or the ratio in which atoms of different elements are combined. This is crucial because the properties of a compound are determined by this specific arrangement and proportion of atoms. A formula allows scientists and students to understand the qualitative and quantitative makeup of a substance without needing to describe it in long, complicated sentences.
A perfect example to illustrate this is the comparison between water (H₂O) and hydrogen peroxide (H₂O₂). While both substances contain only hydrogen and oxygen atoms, their formulas show a critical difference. Hydrogen peroxide, however, has two hydrogen atoms for every two oxygen atoms (a 1:1 ratio). This seemingly small difference in atomic composition, clearly shown by their formulas, results in vastly different properties. Water is essential for life and stable, whereas hydrogen peroxide is a powerful bleaching agent and disinfectant. This clearly demonstrates how a chemical formula defines a substance’s unique identity.
Question 10.
1. What do you understand by the following terms? Acid radical
2. What do you understand by the following terms? Basic radical
Ans:
1. Acid Radical
An acid radical, often referred to as an anion, is the negatively charged component that remains after a hydrogen ion (H⁺) has been removed from an acid.
Detailed Explanation:
In chemistry, many acids are compounds that can release a hydrogen ion (H⁺). When this H⁺ is removed, the leftover part of the molecule carries a negative charge. This negatively charged ion is the acid radical.
- Formation: It is formed during chemical reactions like dissociation. For example, when sulfuric acid (H₂SO₄) dissociates in water, it can lose H⁺ ions to form the sulfate radical (SO₄²⁻).
- Role in Salts: In a salt, the acid radical is the partner to the basic radical. For instance, in common table salt, Sodium Chloride (NaCl), the Chloride ion (Cl⁻) is the acid radical that originated from hydrochloric acid (HCl).
- Key Characteristic: It is always an anion (a negatively charged ion).
- Examples:
- From Hydrochloric Acid (HCl) → Chloride (Cl⁻)
- From Nitric Acid (HNO₃) → Nitrate (NO₃⁻)
- From Carbonic Acid (H₂CO₃) → Carbonate (CO₃²⁻)
In essence, the acid radical is the “acidic part” of a molecule that gives a compound many of its characteristic properties, especially when dissolved in water.
2. Basic Radical
A basic radical, commonly known as a cation, is the positively charged ion that is formed from a base and functions as the central, positively charged part of a salt molecule.
Detailed Explanation:
A basic radical is the metallic (or sometimes ammonium, NH₄⁺) part that is left when the base is involved in salt formation.
- Formation: It often comes from the metal atom in a base. For example, when sodium hydroxide (NaOH) dissociates, it releases the sodium ion (Na⁺), which is a basic radical.
- Role in Salts: In any salt, the basic radical is the positively charged ion. In Copper Sulfate (CuSO₄), the Copper ion (Cu²⁺) is the basic radical.
- Key Characteristic: It is always a cation (a positively charged ion).
- Examples:
- From Sodium Hydroxide (NaOH) → Sodium (Na⁺)
- From Potassium Hydroxide (KOH) → Potassium (K⁺)
- From Calcium Hydroxide (Ca(OH)₂) → Calcium (Ca²⁺)
- From Ammonium Hydroxide (NH₄OH) → Ammonium (NH₄⁺)
Question 11.
1.Select the basic and acidic radicals in the following compounds. MgSO4
2. Select the basic and acidic radicals in the following compounds. (NH4)2SO4
3. Select the basic and acidic radicals in the following compounds. Al2(SO4)3
4. Select the basic and acidic radicals in the following compounds. ZnCO3
5. Select the basic and acidic radicals in the following compounds. Mg(OH)2
Ans:
1. MgSO₄
- Basic Radical: Magnesium (Mg²⁺)
- Acidic Radical: Sulphate (SO₄²⁻)
- Explanation: The magnesium ion carries the positive charge, making it the basic radical, while the sulphate group is the negatively charged acidic radical.
2. (NH₄)₂SO₄
- Basic Radical: Ammonium (NH₄⁺)
- Acidic Radical: Sulphate (SO₄²⁻)
- Explanation: In this case, the ammonium group itself acts as the positively charged basic radical, which is paired with the sulphate acidic radical.
3. Al₂(SO₄)₃
- Basic Radical: Aluminium (Al³⁺)
- Acidic Radical: Sulphate (SO₄²⁻)
- Explanation: The aluminium ion is the positive, basic part of the compound, and the sulphate ion is the negative, acidic part.
4. ZnCO₃
- Basic Radical: Zinc (Zn²⁺)
- Acidic Radical: Carbonate (CO₃²⁻)
- Explanation: The zinc ion forms the basic radical, and the carbonate ion serves as the acidic radical in this compound.
5. Mg(OH)₂
- Basic Radical: Magnesium (Mg²⁺)
- Acidic Radical: Hydroxide (OH⁻)
- Explanation: Here, the magnesium ion is the basic radical, while the hydroxide group functions as the negatively charged acidic radical.
Question 12.
Write a chemical formula of the sulphate of Aluminium, Ammonium and Zinc.
Ans:
The sulphate ion carries a charge of -2 (SO₄²⁻). To find the formula of a sulphate compound, we balance this charge with the positive ion.
- For Aluminium, the ion is Al³⁺. To balance the -2 charge of one sulphate ion, we need a ratio that gives a total positive charge of +2. This is achieved by taking two Al³⁺ ions (total +6 charge) and three SO₄²⁻ ions (total -6 charge). Therefore, the chemical formula is Al₂(SO₄)₃.
- For Ammonium, the ion is NH₄⁺. Since it has a +1 charge, it takes two ammonium ions to balance the -2 charge of one sulphate ion. This gives us the formula (NH₄)₂SO₄. The parentheses are used to show that the subscript 2 applies to the entire NH₄ unit.
- For Zinc, the ion is Zn²⁺. It has a +2 charge, which directly balances the -2 charge of a single sulphate ion. This results in a simple one-to-one ratio, giving the formula ZnSO₄.
Question 13.
The valency of element A is 3 and that of element B is 2. Write the formula of the compound formed by the combination of A and B.
Ans:
The valency of an element indicates its combining power, which determines how many atoms of another element it can bond with. In this case, element A has a valency of 3, meaning it can accept or share 3 electrons. Element B has a valency of 2, meaning it can accept or share 2 electrons.
For these two elements to form a stable compound, the total positive valency must equal the total negative valency. To achieve this electrical balance, two atoms of A (with a total valency of 2 × 3 = 6) will combine with three atoms of B (with a total valency of 3 × 2 = 6). This criss-cross of their valencies ensures neutrality.
Therefore, the chemical formula for the compound formed by the combination of A and B is A₂B₃.
Question 14.
Match the following:
| Compound | Formula |
| 1. Boric acid | i. NaOH |
| 2. Phosphoric acid | ii. SiO2 |
| 3. Nitrous acid | iii. Na2CO3 |
| 4. Nitric acid | iv. KOH |
| 5. Sulphurous acid | v. CaCO3 |
| 6. Sulphuric acid | vi. NaHCO3 |
| 7. Hydrochloric acid | vii. H2S |
| 8. Silica (sand) | viii. H2O |
| 9. Caustic soda (sodium hydroxide) | ix. PH3 |
| 10. Caustic potash (potassium hydroxide) | x. CH4 |
| 11. Washing soda (sodium carbonate) | xi. NH3 |
| 12. Baking soda (sodium bicarbonate) | xii. HCl |
| 13. Lime stone. (calcium carbonate) | xiii. H2SO3 |
| 14. Water | xiv. HNO3 |
| 15. Hydrogen sulphide | xv. HNO2 |
| 16. Ammonia | xvi. H3BO3 |
| 17. Phosphine | xvii. H3PO4 |
| 18. Methane | xviii. H2SO4 |
Ans:
| Compound | Match | Formula |
| 1. Boric acid | xvi | H3BO3 |
| 2. Phosphoric acid | xvii | H3PO4 |
| 3. Nitrous acid | xv | HNO2 |
| 4. Nitric acid | xiv | HNO3 |
| 5. Sulphurous acid | xiii | H2SO3 |
| 6. Sulphuric acid | xviii | H2SO4 |
| 7. Hydrochloric acid | xii | HCl |
| 8. Silica (sand) | ii | SiO2 |
| 9. Caustic soda (sodium hydroxide) | i | NaOH |
| 10. Caustic potash (potassium hydroxide) | iv | KOH |
| 11. Washing soda (sodium carbonate) | iii | Na2CO3 |
| 12. Baking soda (sodium bicarbonate) | vi | NaHCO3 |
| 13. Lime stone. (calcium carbonate) | v | CaCO3 |
| 14. Water | viii | H2O |
| 15. Hydrogen sulphide | vii | H2S |
| 16. Ammonia | xi | NH3 |
| 17. Phosphine | ix | PH3 |
| 18. Methane | x | CH4 |
Question 15.
1. Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compounds. Barium sulphate
2. Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compounds. Potassium ferrocyanide
3. Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compounds. Bismuth nitrate
4. Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compounds. Calcium bromide
5. Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compounds. Ferrous sulphide
6. Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compounds. Chromium sulphate
7. Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compounds. Calcium silicate
8. Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compounds. Stannic oxide
9. Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compounds. Sodium zincate
10. Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compounds. Magnesium phosphate
11. Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compounds. Sodium thiosulphate
12. Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compounds. Stannic phosphate
13. Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compounds.Nickel bisulphate
14. Write the basic radicals and acidic radicals of the following and then write the chemical formulae of these compounds. Potassium manganate
Ans:
| No. | Compound Name | Basic Radical (Cation) | Acidic Radical (Anion) | Chemical Formula |
| 1 | Barium sulphate | Ba2+ (Valency 2) | SO42− (Valency 2) | BaSO4 |
| 2 | Potassium ferrocyanide | K+ (Valency 1) | [Fe(CN)6]4− (Valency 4) | K4[Fe(CN)6] |
| 3 | Bismuth nitrate | Bi3+ (Valency 3) | NO3− (Valency 1) | Bi(NO3)3 |
| 4 | Calcium bromide | Ca2+ (Valency 2) | Br− (Valency 1) | CaBr2 |
| 5 | Ferrous sulphide | Fe2+ (Valency 2) | S2− (Valency 2) | FeS |
| 6 | Chromium sulphate | Cr3+ (Valency 3) | SO42− (Valency 2) | Cr2(SO4)3 |
| 7 | Calcium silicate | Ca2+ (Valency 2) | SiO32− (Valency 2) | CaSiO3 |
| 8 | Stannic oxide | Sn4+ (Valency 4) | O2− (Valency 2) | SnO2 |
| 9 | Sodium zincate | Na+ (Valency 1) | ZnO22− (Valency 2) | Na2ZnO2 |
| 10 | Magnesium phosphate | Mg2+ (Valency 2) | PO43− (Valency 3) | Mg3(PO4)2 |
| 11 | Sodium thiosulphate | Na+ (Valency 1) | S2O32− (Valency 2) | Na2S2O3 |
| 12 | Stannic phosphate | Sn4+ (Valency 4) | PO43− (Valency 3) | Sn3(PO4)4 |
| 13 | Nickel bisulphate | Ni2+ (Valency 2) | HSO4− (Valency 1) | Ni(HSO4)2 |
| 14 | Potassium manganate | K+ (Valency 1) | MnO42− (Valency 2) | K2MnO4 |
Question 16.
1. Write the chemical name of the following compound: Ca3(PO4)2
2. Write the chemical name of the following compound: K2CO3
3. Write the chemical name of the following compound: K2MnO4
4. Write the chemical name of the following compound: Mn3(BO3)2
5. Write the chemical name of the following compound: Mg(HCO3)2
6. Write the chemical name of the following compound: Na4Fe(CN)6
7. Write the chemical name of the following compound: Ba(ClO3)2
8. Write the chemical name of the following compound: Ag2SO3
9. Write the chemical name of the following compound: (CH3COO)2Pb
10. Write the chemical name of the following compound: Na2SiO3
Ans:
- Ca₃(PO₄)₂ is named Calcium Phosphate. This compound is well-known as a major mineral component of bones and teeth.
- K₂CO₃ is called Potassium Carbonate. It is commonly known as pearl ash and is used in the production of soap and glass.
- K₂MnO₄ is Potassium Manganate. It is recognized by its distinctive bright green color in chemical solutions.
- Mn₃(BO₃)₂ is named Manganese(II) Borate. The Roman numeral (II) specifies the charge of the manganese ion in this compound.
- Mg(HCO₃)₂ is Magnesium Bicarbonate. This is the temporary hardness salt found in hard water, which decomposes upon heating.
- Na₄Fe(CN)₆ is Sodium Ferrocyanide. It is a yellow crystalline solid and is one of the main cyanide compounds used in industry.
- Ba(ClO₃)₂ is Barium Chlorate. This compound is often used in pyrotechnics to produce a vibrant green colored flame.
- Ag₂SO₃ is Silver Sulfite. It is a light-sensitive compound, which is typical for many silver-based chemicals.
- (CH₃COO)₂Pb is Lead(II) Acetate. Historically, it was called “sugar of lead” due to its sweet taste, but it is highly toxic.
- Na₂SiO₃ is Sodium Silicate. It is popularly known as water glass or liquid glass because its solutions are used as a sealant.
Question 17.
1. Give the name of the following compound : KCIO
2. Give the name of the following compound: KClO2
3. Give the name of the following compound: KClO3
4. Give the name of the following compound: KClO4
Ans:
Here are the names for the compounds, based on the standard rules for naming inorganic compounds.
- KClO is potassium hypochlorite.
- Reasoning: The polyatomic ion ClO⁻ is the hypochlorite ion.
- KClO₂ is potassium chlorite.
- Reasoning: The polyatomic ion ClO₂⁻ is the chlorite ion.
- KClO₃ is potassium chlorate.
- Reasoning: The polyatomic ion ClO₃⁻ is the chlorate ion.
- KClO₄ is potassium perchlorate.
- Reasoning: The polyatomic ion ClO₄⁻ is the perchlorate ion.
Question 18.
1.Complete the following statement by selecting the correct option : The formula of a compound represents
- an atom
- a particle
- a molecule
2. Complete the following statement by selecting the correct option : The correct formula of aluminium oxide is
- AlO3
- AlO2
- Al2O3
3. Complete the following statement by selecting the correct option : The valency of nitrogen in nitrogen dioxide (NO2) is
- one
- two
- three
- Four
Question 19.
1. Give the name of the element and number of atoms of those elements present in the following compound. Sodium sulphate
2. Give the name of the element and number of atoms of those elements present in the following compound. Quick lime
3. Give the name of the element and number of atoms of those elements present in the following compound. Baking soda (NaHCO3)
4. Give the name of the element and number of atoms of those elements present in the following compound. Ammonia
5. Give the name of the element and number of atoms of those elements present in the following compound. Ammonium dichromate
Ans:
1. Sodium Sulphate (Na₂SO₄)
The compound Sodium Sulphate is composed of three distinct elements. It contains the elements Sodium (Na), Sulphur (S), and Oxygen (O). In one molecule of this compound, there are 2 atoms of Sodium, 1 atom of Sulphur, and 4 atoms of Oxygen.
2. Quick Lime (CaO)
Quick Lime has a very simple chemical structure. It is made up of only two elements: Calcium (Ca) and Oxygen (O). Therefore, a single molecule of Quick Lime contains 1 atom of Calcium and 1 atom of Oxygen.
3. Baking Soda (NaHCO₃)
The compound known as Baking Soda consists of four different elements. These are Sodium (Na), Hydrogen (H), Carbon (C), and Oxygen (O). The molecule includes 1 atom of Sodium, 1 atom of Hydrogen, 1 atom of Carbon, and 3 atoms of Oxygen.
4. Ammonia (NH₃)
Ammonia is a compound formed from the elements Nitrogen (N) and Hydrogen (H). In each molecule of Ammonia, you will find 1 atom of Nitrogen and 3 atoms of Hydrogen.
5. Ammonium Dichromate ((NH₄)₂Cr₂O₇)
This compound has a more complex structure. The elements present are Nitrogen (N), Hydrogen (H), Chromium (Cr), and Oxygen (O). The small ‘4’ and ‘2’ outside the parentheses mean we multiply everything inside. This gives us a total of 2 atoms of Nitrogen, 8 atoms of Hydrogen, 2 atoms of Chromium, and 7 atoms of Oxygen.
Question 20.
1.The formula of the sulphate of an element M is M2(SO4) Write the formula of it Chloride
2. The formula of the sulphate of an element M is M2(SO4)3 Write the formula of it Oxide
3. The formula of the sulphate of an element M is M2(SO4)3 Write the formula of it Phosphate
4. The formula of the sulphate of an element M is M2(SO4)3 Write the formula of its Acetate
Ans:
1. The formula of the sulphate of an element M is M₂(SO₄). Write the formula of its Chloride.
- Solution:
- The sulphate ion is (SO₄)²⁻.
- In M₂(SO₄), two atoms of M combine with one (SO₄)²⁻ ion.
- This means the total positive charge from two M atoms is +2. Therefore, the charge (valency) of one M atom is +1.
- The chloride ion is Cl⁻.
- To form a neutral compound, one M⁺ ion will combine with one Cl⁻ ion.
- Final Answer: MCl
2. The formula of the sulphate of an element M is M₂(SO₄)₃. Write the formula of its Oxide.
- Solution:
- The sulphate ion is (SO₄)²⁻.
- In M₂(SO₄)₃, two atoms of M combine with three (SO₄)²⁻ ions.
- The total negative charge is 3 × (-2) = -6.
- Therefore, the total positive charge from two M atoms must be +6. The charge (valency) of one M atom is +3.
- The oxide ion is O²⁻.
- To form a neutral compound, the charges must balance. Two M³⁺ ions have a total charge of +6, and three O²⁻ ions have a total charge of -6.
- Final Answer: M₂O₃
3. The formula of the sulphate of an element M is M₂(SO₄)₃. Write the formula of its Phosphate.
- Solution:
- As established in question 2, the valency of M is +3.
- The phosphate ion is (PO₄)³⁻.
- To form a neutral compound, the +3 charge from one M³⁺ ion directly balances the -3 charge from one (PO₄)³⁻ ion.
- Final Answer: MPO₄
4. The formula of the sulphate of an element M is M₂(SO₄)₃. Write the formula of its Acetate.
- Solution:
- As established in question 2, the valency of M is +3.
- The acetate ion is (CH₃COO)⁻. (It is often written as C₂H₃O₂⁻).
- To form a neutral compound, one M³⁺ ion requires three acetate ions (each with a -1 charge) to balance the charge.
- Final Answer: M(CH₃COO)₃ or M(C₂H₃O₂)₃
Exercise 1 (B)
Question 1.
What is a chemical equation? Why is it necessary to balance it?
Ans:
A chemical equation is like a precise recipe for a chemical reaction. It uses symbols and formulas instead of words to show the substances you start with, called reactants, and the new substances that are formed, called products. For instance, the burning of charcoal can be written as: Carbon + Oxygen → Carbon Dioxide, or more scientifically as C + O₂ → CO₂. The arrow signifies “yields” or “produces,” pointing from the starting materials to the final result. In essence, it is a concise shorthand that scientists use to describe and communicate the transformation of matter at a molecular level.
It is absolutely necessary to balance this chemical equation because of a fundamental law of nature: the Law of Conservation of Mass. This law states that matter cannot be created or destroyed in an ordinary chemical reaction. Think of it like rearranging building blocks—you can’t end up with more blocks than you started with, and you can’t have any blocks disappear. The atoms present at the beginning must all be accounted for in the end; they are simply rearranged into new combinations.
Therefore, balancing the equation is the process of ensuring that the number of each type of atom on the reactant side is exactly equal to the number on the product side. We do this by placing numbers, called coefficients, in front of the chemical formulas. We never change the formulas themselves, as that would create a different substance. A balanced equation like 2H₂ + O₂ → 2H₂O confirms that four hydrogen atoms and two oxygen atoms are both the input and the output of the reaction, faithfully obeying the Law of Conservation of Mass and giving a true picture of the chemical change.
Question 2.
State the information conveyed by the following equation: Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2 ↑
Ans:
Here is the information conveyed by the chemical equation Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g):
This chemical equation provides a concise and informative summary of a chemical reaction, conveying the following specific details:
- Identities of the Reactants and Products:
- The reaction begins with solid zinc metal (Zn(s)) and an aqueous solution of hydrochloric acid (2HCl(aq)).
- It produces an aqueous solution of zinc chloride (ZnCl₂(aq)) and hydrogen gas (H₂(g)).
- Physical States of all Substances:
- (s) denotes that zinc is a solid.
- (aq) denotes that HCl and ZnCl₂ are dissolved in water, forming an aqueous solution.
- (g) or the upward arrow (↑) indicates that hydrogen is a gas being released from the mixture.
- Stoichiometry of the Reaction (Mole Ratios):
- The coefficients indicate the exact proportions in which the molecules react and are formed.
- 1 mole of zinc metal reacts with 2 moles of hydrochloric acid to produce 1 mole of zinc chloride and 1 mole of hydrogen gas.
- The Fundamental Nature of the Reaction:
- This is a classic example of a single displacement reaction (also called a substitution reaction). The more reactive zinc metal displaces or “pushes out” the hydrogen from the acid.
- It is also an example of a redox reaction (reduction-oxidation). Zinc metal is oxidized, losing electrons to form Zn²⁺ ions, while the hydrogen in HCl is reduced, gaining electrons to form H₂ gas.
- Observable Phenomenon:
- From a practical, laboratory perspective, the equation describes the observable event where a piece of zinc metal bubbles vigorously when placed in hydrochloric acid. The bubbles are the hydrogen gas being evolved. The solid zinc will eventually dissolve completely.
Question 3.
What is the limitation of the reaction given in question 2?
Ans:
While a balanced chemical equation is a vital tool, it paints an idealized picture that often clashes with laboratory reality. The most significant shortcoming is its suggestion that reactants transform into products entirely. In practice, many reactions are reversible, stalling at a point of equilibrium where reactants and products coexist. This dynamic balance means that a portion of the starting materials is never consumed, guaranteeing that the amount of product you actually obtain will fall short of the theoretical amount predicted by the formula’s neat ratios.
Beyond the issue of completion, the equation is silent on the practical “how-to” that a chemist needs. It provides no guidance on the specific environmental conditions required to make the reaction feasible or efficient. Critical factors like the necessary temperature, a specific pressure, or the need for a catalyst to speed up the process are completely absent from the simple notation. Without this essential context, a reaction that looks perfect on paper might proceed at an imperceptibly slow rate or fail to initiate altogether in a real-world setting.
Question 4.
1. Write the chemical equation for the following word equation and balance them.
Carbon + Oxygen → Carbon dioxide
2. Write the chemical equation for the following word equation and balance them.
Nitrogen + Oxygen → Nitrogen monoxide
3. Write the chemical equation for the following word equation and balance them.
Calcium + Nitrogen → Calcium nitride
4. Write the chemical equation for the following word equation and balance them.
Calcium oxide + Carbon dioxide → Calcium carbonate
5. Write the chemical equation for the following word equation and balance them.
Magnesium + Sulphuric acid → Magnesium sulphate + Hydrogen
6. Write the chemical equation for the following word equation and balance them.
Sodium reacts with water to form sodium hydroxide and hydrogen
Ans:
- Carbon reacts with oxygen to produce carbon dioxide.
- Chemical Equation: C + O₂ → CO₂
- Reasoning: This reaction is naturally balanced from the start. A single carbon atom combines with a molecule of oxygen (O₂) to form one molecule of carbon dioxide (CO₂), so the number of each type of atom is identical on both sides.
- Nitrogen and oxygen combine to form nitrogen monoxide.
- Chemical Equation: N₂ + O₂ → 2NO
- Reasoning: Since nitrogen and oxygen gases exist as pairs of atoms (N₂ and O₂), we start with these diatomic molecules. To have the same number of atoms on both sides, the reaction must produce two molecules of nitrogen monoxide (NO).
- Calcium metal reacts with nitrogen gas to yield calcium nitride.
- Chemical Equation: 3Ca + N₂ → Ca₃N₂
- Reasoning: The product, calcium nitride, has the fixed formula Ca₃N₂, meaning it contains three calcium atoms for every two nitrogen atoms. To achieve this ratio, we need three atoms of calcium and two atoms of nitrogen as reactants. Using the diatomic N₂ molecule provides the two nitrogen atoms, so we balance it with three individual calcium atoms.
- Calcium oxide and carbon dioxide combine to create calcium carbonate.
- Chemical Equation: CaO + CO₂ → CaCO₃
- Reasoning: This is a straightforward combination reaction where one molecule of calcium oxide and one molecule of carbon dioxide directly form one molecule of calcium carbonate. The atom count is already equal on both sides of the equation.
- Magnesium displaces hydrogen from sulfuric acid to form magnesium sulfate and hydrogen gas.
- Chemical Equation: Mg + H₂SO₄ → MgSO₄ + H₂
- Reasoning: In this single displacement reaction, one magnesium atom replaces the two hydrogen atoms in sulfuric acid. This swap results in one molecule of magnesium sulfate and one molecule of diatomic hydrogen gas (H₂), making the equation balanced as written.
- Sodium metal reacts vigorously with water, producing sodium hydroxide and hydrogen gas.
- Chemical Equation: 2Na + 2H₂O → 2NaOH + H₂
- Reasoning: Balancing this reaction requires a slightly larger scale. Two sodium atoms are needed to react with two water molecules. This produces two units of sodium hydroxide and one molecule of hydrogen gas (H₂), which properly accounts for all the hydrogen and oxygen atoms.
Question 5.
1. Balance the following equation: Fe + H2O → Fe3O4 + H2
2. Balance the following equation: Ca + N2 → Ca3N2
3. Balance the following equation: Zn + KOH → K2ZnO2 + H2
4. Balance the following equation: Fe2O3 + CO → Fe + CO2
5. Balance the following equation: PbO + NH3 → Pb + H2O + N2
6. Balance the following equation: Pb3O4 → PbO + O2
7. Balance the following equation: PbS + O2 → PbO + SO2
8. Balance the following equation: S + H2SO4 → SO2 + H2O
9. Balance the following equation: S+HNO3→ H2SO4+NO2+H2O
10. Balance the following equation: MnO2 + HCl → MnCl2 + H2O + Cl2
11. Balance the following equation: C + H2SO4 → CO2 + H2O + SO2
12. Balance the following equation: KOH + Cl2 → KCl + KClO + H2O
13. Balance the following equation: NO2 +H2O → HNO2 + HNO3
14. Balance the following equation: Pb3O4 + HCl → PbCl2 + H2O + Cl2
15. Balance the following equation: H2O + Cl2 → HCl + O2
16. Balance the following equation: NaHCO3 → Na2CO3 + H2O + CO2
17. Balance the following equation: HNO3 + H2S → NO2 + H2O + S
18. Balance the following equation:P + HNO3 → NO2 + H2O + H3PO4
19. Balance the following equation: Zn + HNO3 → Zn(NO3)2 + H2O + NO2
Ans:
1. Iron with Steam
To balance the reaction between iron and steam, we start with the skeleton equation: Fe + H₂O → Fe₃O₄ + H₂. First, balance the iron atoms by placing a 3 before Fe. Next, balance the oxygen atoms; since Fe₃O₄ has four oxygen atoms, we need four water molecules. This gives us 3Fe + 4H₂O → Fe₃O₄ + H₂. Finally, the four water molecules contribute eight hydrogen atoms, requiring four hydrogen molecules on the product side. The final balanced equation is 3Fe + 4H₂O → Fe₃O₄ + 4H₂.
2. Calcium with Nitrogen
For the formation of calcium nitride, Ca + N₂ → Ca₃N₂, we see three calcium atoms are needed on the reactant side to match the product. Placing a 3 before Ca gives 3Ca + N₂ → Ca₃N₂. The nitrogen is already balanced with two atoms on each side. The balanced equation is 3Ca + N₂ → Ca₃N₂.
3. Zinc with Potassium Hydroxide
The reaction Zn + KOH → K₂ZnO₂ + H₂ requires balancing potassium first. Placing a 2 before KOH gives Zn + 2KOH → K₂ZnO₂ + H₂. This also balances the hydrogen and oxygen, as the two KOH molecules provide the correct number of atoms for the product side. The balanced equation is Zn + 2KOH → K₂ZnO₂ + H₂.
4. Iron(III) Oxide with Carbon Monoxide
In the reduction of iron ore: Fe₂O₃ + CO → Fe + CO₂, we first balance iron by placing a 2 before Fe. Then, to balance the three oxygen atoms from the ferric oxide, we need three CO molecules, which produce three CO₂ molecules. The balanced equation is Fe₂O₃ + 3CO → 2Fe + 3CO₂.
5. Lead(II) Oxide with Ammonia
This is a redox reaction: PbO + NH₃ → Pb + H₂O + N₂. Balancing the atoms and the electron transfer shows that three molecules of lead oxide react with two molecules of ammonia. The final balanced equation is 3PbO + 2NH₃ → 3Pb + 3H₂O + N₂.
6. Decomposition of Lead(II,IV) Oxide
For the decomposition Pb₃O₄ → PbO + O₂, we start with two molecules of the reactant to balance the lead atoms: 2Pb₃O₄ → 6PbO + O₂. This also balances the oxygen, with eight atoms on each side. The balanced equation is 2Pb₃O₄ → 6PbO + O₂.
7. Roasting of Lead Sulphide
In the roasting process: PbS + O₂ → PbO + SO₂, we begin with two molecules of lead sulphide. This requires two molecules of sulphur dioxide and three molecules of oxygen to balance all atoms. The balanced equation is 2PbS + 3O₂ → 2PbO + 2SO₂.
8. Sulfur with Concentrated Sulfuric Acid
This disproportionation reaction, S + H₂SO₄ → SO₂ + H₂O, requires two molecules of acid to balance the sulfur, hydrogen, and oxygen atoms. The balanced equation is S + 2H₂SO₄ → 3SO₂ + 2H₂O.
9. Sulfur with Concentrated Nitric Acid
For the reaction S + HNO₃ → H₂SO₄ + NO₂ + H₂O, six molecules of nitric acid are needed to provide the oxygen for the oxidation of sulfur to sulfuric acid and to form nitrogen dioxide. The balanced equation is S + 6HNO₃ → H₂SO₄ + 6NO₂ + 2H₂O.
10. Manganese Dioxide with Hydrochloric Acid
In this lab preparation of chlorine: MnO₂ + HCl → MnCl₂ + H₂O + Cl₂, four molecules of hydrochloric acid are needed to provide the chlorine and hydrogen atoms. The balanced equation is MnO₂ + 4HCl → MnCl₂ + 2H₂O + Cl₂.
11. Carbon with Concentrated Sulfuric Acid
The redox reaction C + H₂SO₄ → CO₂ + H₂O + SO₂ requires two molecules of sulfuric acid to balance the atoms involved in the oxidation of carbon. The balanced equation is C + 2H₂SO₄ → CO₂ + 2H₂O + 2SO₂.
12. Chlorine with Cold Potassium Hydroxide
This disproportionation reaction, KOH + Cl₂ → KCl + KClO + H₂O, needs two molecules of potassium hydroxide to balance the potassium, hydrogen, and oxygen. The balanced equation is 2KOH + Cl₂ → KCl + KClO + H₂O.
13. Nitrogen Dioxide with Water
Another disproportionation reaction, NO₂ + H₂O → HNO₂ + HNO₃, requires two molecules of nitrogen dioxide to form the two different acids. The balanced equation is 2NO₂ + H₂O → HNO₂ + HNO₃.
14. Lead(II,IV) Oxide with Hydrochloric Acid
In this reaction, Pb₃O₄ + HCl → PbCl₂ + H₂O + Cl₂, eight HCl molecules are needed to react with the oxide, releasing chlorine gas. The balanced equation is Pb₃O₄ + 8HCl → 3PbCl₂ + 4H₂O + Cl₂.
15. Water with Chlorine
In sunlight, the reaction H₂O + Cl₂ → HCl + O₂ proceeds. To balance, we need two molecules of water and two of chlorine to produce hydrochloric acid and oxygen. The balanced equation is 2H₂O + 2Cl₂ → 4HCl + O₂.
16. Decomposition of Sodium Bicarbonate
Upon heating, sodium bicarbonate decomposes: NaHCO₃ → Na₂CO₃ + H₂O + CO₂. Two molecules of the reactant are needed to balance the sodium atoms and all other elements. The balanced equation is 2NaHCO₃ → Na₂CO₃ + H₂O + CO₂.
17. Hydrogen Sulfide with Nitric Acid
For the reaction HNO₃ + H₂S → NO₂ + H₂O + S, two molecules of nitric acid are required to oxidize the hydrogen sulfide. The balanced equation is 2HNO₃ + H₂S → 2NO₂ + 2H₂O + S.
18. Phosphorus with Nitric Acid
The vigorous reaction P + HNO₃ → NO₂ + H₂O + H₃PO₄ requires five molecules of concentrated nitric acid to fully oxidize phosphorus. The balanced equation is P + 5HNO₃ → 5NO₂ + H₂O + H₃PO₄.
19. Zinc with Concentrated Nitric Acid
With concentrated acid, zinc reacts: Zn + HNO₃ → Zn(NO₃)₂ + H₂O + NO₂. Four molecules of nitric acid are needed, with one nitrogen atom being reduced to nitrogen dioxide. The balanced equation is Zn + 4HNO₃ → Zn(NO₃)₂ + 2H₂O + NO₂.
Exercise 1 (C)
Question 1.
1. Fill in the blank: Dalton used the symbol _____ for oxygen and _____ for hydrogen.
2. Fill in the blank: Symbol represents _____ atom(s) of an element.
3. Fill in the blank: Symbolic expression for a molecule is called _____.
4. Fill in the blank: Sodium chloride has two radicals. Sodium is a _____ radical while chloride is _____ radical.
5. Fill in the blank: Valency of carbon in CH4 is _____ , in C2H6 _____, in C2H4 ___ and in C2H2 is ____.
6. Fill in the blank:Valency of Iron in FeCl2 is _____ and in FeCl3 it is ____ .
7. Fill in the blank: The formula of iron (ill) carbonate is _____ .
Ans:
- Fill in the blank: Dalton used the symbol ● for oxygen and ○ for hydrogen.
(Note: Dalton’s symbols were pictorial. A circle with a center dot was oxygen, and a hollow circle was hydrogen.) - Fill in the blank: Symbol represents one atom(s) of an element.
- Fill in the blank: Symbolic expression for a molecule is called a molecular formula.
- Fill in the blank: Sodium chloride has two radicals. Sodium is a basic radical while chloride is an acidic radical.
- Fill in the blank: Valency of carbon in CH₄ is four , in C₂H₆ four, in C₂H₄ two and in C₂H₂ is two.
- Fill in the blank: Valency of Iron in FeCl₂ is two and in FeCl₃ it is three.
- Fill in the blank: The formula of iron (III) carbonate is Fe₂(CO₃)₃.
Question 2.
Complete the following table.
| Acid Radicals → | Chloride | Nitrate | Sulphate | Carbonate | Hydroxide | Phosphate |
| Basic Radicals ↓ | ||||||
| Magnesium | MgCl2 | Mg(NO3)2 | MgSO4 | MgCO3 | Mg(OH)2 | Mg3(PO4)2 |
| Sodium | ||||||
| Zinc | ||||||
| Silver | ||||||
| Ammonium | ||||||
| Calcium | ||||||
| Iron (II) | ||||||
| Potassium |
Ans:
| Basic Radicals ↓ | Chloride (Cl−) | Nitrate (NO3−) | Sulphate (SO42−) | Carbonate (CO32−) | Hydroxide (OH−) | Phosphate (PO43−) |
| Magnesium (Mg2+) | MgCl2 | Mg(NO3)2 | MgSO4 | MgCO3 | Mg(OH)2 | Mg3(PO4)2 |
| Sodium (Na+) | NaCl | NaNO3 | Na2SO4 | Na2CO3 | NaOH | Na3PO4 |
| Zinc (Zn2+) | ZnCl2 | Zn(NO3)2 | ZnSO4 | ZnCO3 | Zn(OH)2 | Zn3(PO4)2 |
| Silver (Ag+) | AgCl | AgNO3 | Ag2SO4 | Ag2CO3 | AgOH | Ag3PO4 |
| Ammonium (NH4+) | NH4Cl | NH4NO3 | (NH4)2SO4 | (NH4)2CO3 | NH4OH | (NH4)3PO4 |
| Calcium (Ca2+) | CaCl2 | Ca(NO3)2 | CaSO4 | CaCO3 | Ca(OH)2 | Ca3(PO4)2 |
| Iron (II) (Fe2+) | FeCl2 | Fe(NO3)2 | FeSO4 | FeCO3 | Fe(OH)2 | Fe3(PO4)2 |
| Potassium (K+) | KCl | KNO3 | K2SO4 | K2CO3 | KOH | K3PO4 |
Question 3.
Sodium chloride reacts with silver nitrate to produce silver chloride and sodium nitrate
Write the equation. Check whether it is balanced, if not balance it. Find the weights of reactants and products. State the law which this equation satisfies.
Ans:
The interaction between sodium chloride and silver nitrate is a classic example of a double displacement reaction, where the ions in the reactants exchange partners. This process is clearly communicated using a chemical equation. We begin by writing the skeletal equation: NaCl + AgNO₃ → AgCl + NaNO₃. This shows that sodium chloride reacts with silver nitrate to produce silver chloride and sodium nitrate.
Before proceeding, it is essential to verify that the equation adheres to the Law of Conservation of Mass, meaning the number of atoms for each element must be the same on both sides. A quick count confirms this: one sodium, one chlorine, one silver, one nitrogen, and three oxygen atoms are present on the reactant and product sides. This confirms the equation is already perfectly balanced as written, with no need for additional numbers or coefficients.
To further validate the conservation of mass, we can examine the molecular weights. Calculating them (using Na=23, Cl=35.5, Ag=108, N=14, O=16), we find NaCl is 58.5 u, AgNO₃ is 170 u, AgCl is 143.5 u, and NaNO₃ is 85 u. Adding the masses of the reactants (58.5 + 170) gives 228.5 u, and adding the masses of the products (143.5 + 85) also gives 228.5 u. This numerical equality provides concrete proof that the total mass remains unchanged, satisfying the fundamental law that governs all chemical changes.
Question 4.
1. What information does the following chemical equation convey? Zn + H2SO4 → ZnSO4+ H2
2. What information do the following chemical equations convey? Mg + 2HCl → MgCl2+ H2
Ans:
1. Information Conveyed by: Zn + H₂SO₄ → ZnSO₄ + H₂
This chemical equation describes a single displacement reaction and provides the following specific information:
- Identities of Reactants and Products: The reaction begins with solid Zinc metal (Zn) and aqueous Sulfuric Acid (H₂SO₄). It results in the formation of aqueous Zinc Sulfate (ZnSO₄) and Hydrogen gas (H₂).
- Reaction Type: This is a classic example of a single displacement reaction. Here, the more reactive zinc metal displaces or pushes out the hydrogen from the acid. The hydrogen, which was part of a compound, is released as a free gas.
- Physical States (Implied): While not explicitly stated, the standard context tells us that Zinc is a solid metal, Sulfuric Acid is an aqueous solution, Zinc Sulfate is formed as an aqueous solution, and Hydrogen is a gaseous product. The evolution of hydrogen gas is often observed as bubbles.
- Conservation of Atoms: The equation is balanced, showing that no atoms are lost or gained. One zinc atom, two hydrogen atoms, one sulfur atom, and four oxygen atoms are present on both sides of the arrow.
- Quantitative Relationships: The coefficients indicate the ratio in which molecules react. One atom of zinc reacts with one molecule of sulfuric acid to produce one unit of zinc sulfate and one molecule of hydrogen gas. This means equal numbers of zinc atoms and sulfuric acid molecules are involved.
2. Information Conveyed by: Mg + 2HCl → MgCl₂ + H₂
This equation also depicts a single displacement reaction but reveals a different quantitative relationship:
- Identities of Reactants and Products: The starting materials are solid Magnesium metal (Mg) and aqueous Hydrochloric Acid (HCl).
- Reaction Type: Similar to the first, this is a single displacement reaction. The magnesium metal, being highly reactive, displaces the hydrogen from the hydrochloric acid, leading to the release of hydrogen gas.
- Physical States (Implied): Magnesium is a solid metal, Hydrochloric Acid is an aqueous solution, Magnesium Chloride is soluble in water (aqueous), and Hydrogen is a gas.
- Crucial Stoichiometric Ratio: A key piece of information here is the coefficient “2” in front of HCl. This shows that a single atom of magnesium requires two molecules of hydrochloric acid for a complete reaction. This is because one magnesium atom can form bonds with two chlorine atoms from two different HCl molecules, releasing two hydrogen atoms which then combine to form one H₂ molecule.
- Conservation of Atoms: The balance of atoms is confirmed: one magnesium atom, two hydrogen atoms, and two chlorine atoms are present on each side of the equation. The “2” in front of HCl is essential for this balance, distinguishing it from the 1:1 ratio in the first equation.
Question 5.
1. What are polyatomic ions? Give two examples.
2. Name the fundamental law that is involved in every equation.
Ans:
- Polyatomic ions are stable, charged species that act as a single unit during chemical reactions. They are formed when a group of two or more atoms are covalently bonded together, but the group as a whole has either gained or lost electrons, resulting in a net positive or negative charge. These ions are crucial because they move and interact with other ions or molecules as a single entity. For example, the Ammonium ion (NH₄⁺), which is positively charged, is common in fertilizers. A common negatively charged example is the Sulphate ion (SO₄²⁻), a key component found in many minerals and salts.
- The fundamental law that is the foundation for every balanced chemical equation is the Law of Conservation of Mass. This law states that mass can neither be created nor destroyed in an ordinary chemical reaction. This is why we must balance chemical equations; the total number of atoms of each element present in the reactants must be exactly equal to the total number of atoms of that same element in the products. This ensures that the mass is conserved from the starting materials to the final substances formed.
Question 6.
1. What is the valency of : fluorine in CaF2
2. What is the valency of : sulphur in SF6
3. What is the valency of : phosphorus in PH3
4. What is the valency of : carbon in CH4
5. What is the valency of : nitrogen in the following compounds: (i) N2O3 (ii) N2O5 (iii) NO2 (iv) NO
6. What is the valency of manganese in MnO2?
7. What is the valency of Copper in Cu2O?
8. What is the valency of : Magnesium in Mg3N2
Ans:
A Guide to Determining Element Valency from Chemical Formulas
A fundamental concept in chemistry is valency, which indicates an element’s combining power. We can deduce this directly from a compound’s chemical formula by remembering a key rule: in a stable compound, the total positive valency must perfectly balance the total negative valency. Here is a breakdown for the listed examples.
1. Fluorine in CaF₂
- Calcium (Ca) consistently has a combining power of 2.
- The formula shows one Ca atom joined with two F atoms.
- For the compound to be stable, the two F atoms together must balance Ca’s valency of 2.
- Conclusion: Each fluorine atom has a valency of 1.
2. Sulphur in SF₆
- Fluorine, as seen above, has a valency of 1.
- Six F atoms bonded to one S atom contribute a total negative valency of 6.
- The single S atom must provide an equal and opposite combining power.
- Conclusion: The sulphur atom has a valency of 6.
3. Phosphorus in PH₃
- Hydrogen (H) has a standard valency of 1.
- Three H atoms create a total positive valency of 3.
- The phosphorus atom balances this with its own combining power.
- Conclusion: The valency of phosphorus here is 3.
4. Carbon in CH₄
- With hydrogen’s valency at 1, four H atoms contribute a total of 4.
- The carbon atom at the center must match this combining capacity.
- Conclusion: Carbon exhibits a valency of 4.
5. Nitrogen in its Oxides
Nitrogen is interesting as it displays multiple valencies.
- In N₂O₃: Three oxygen atoms (each with a valency of 2) contribute a total of 6. This is shared by two nitrogen atoms, so each nitrogen has a valency of 3.
- In N₂O₅: Five oxygen atoms contribute a total valency of 10. Shared between two nitrogen atoms, this gives each nitrogen a valency of 5.
- In NO₂: Two oxygen atoms contribute a total of 4. A single nitrogen atom balances this, showing a valency of 4.
- In NO: One oxygen atom has a valency of 2. The single nitrogen atom matching it shows a valency of 2.
6. Manganese in MnO₂
- Oxygen’s valency is 2.
- Two oxygen atoms contribute a total negative valency of 4.
- The single manganese atom provides the balancing combining power.
- Conclusion: The valency of manganese is 4.
7. Copper in Cu₂O
- Oxygen’s valency is 2.
- This valency of 2 is balanced not by one, but by two copper atoms.
- Therefore, the combining power of each copper atom is 2 ÷ 2.
- Conclusion: The valency of copper is 1.
8. Magnesium in Mg₃N₂
- Nitrogen, from Group 15, commonly has a valency of 3.
- Two nitrogen atoms together have a combining power of 6.
- This is balanced by three magnesium atoms, meaning each one contributes equally.
- Conclusion: The valency of magnesium is 2 (as 6 ÷ 3 = 2).
Question 7.
Why should an equation be balanced? Explain with the help of a simple equation.
Ans:
A chemical equation must be balanced to uphold a fundamental law of nature: the Law of Conservation of Mass. This scientific law states that matter can neither be created nor destroyed in an ordinary chemical reaction. In simpler terms, the total mass and number of atoms of each element we start with (the reactants) must be exactly equal to the total mass and number of atoms we end up with (the products). Atoms are simply rearranged to form new substances; they do not magically appear or vanish.
Let’s understand this with the simple formation of water: Hydrogen gas (H₂) reacts with Oxygen gas (O₂) to form Water (H₂O). If we write this initially as:
H₂ + O₂ → H₂O
This equation is unbalanced. A quick count of atoms reveals the problem. On the left, we have 2 Hydrogen atoms and 2 Oxygen atoms. On the right, we have 2 Hydrogen atoms but only 1 Oxygen atom. An oxygen atom has gone missing, which violates the Law of Conservation of Mass.
To correct this, we balance the equation by placing numbers called coefficients in front of the formulas. We cannot change the formula H₂O itself, as that would represent a different substance. By placing a ‘2’ before H₂O and a ‘2’ before H₂, we get:
2H₂ + O₂ → 2H₂O
Now, let’s count the atoms again. On the left: 4 Hydrogen atoms (2×2) and 2 Oxygen atoms. On the right: 4 Hydrogen atoms (2×2) and 2 Oxygen atoms (2×1). The atom count is now equal on both sides. The equation is balanced, accurately showing that two molecules of hydrogen combine with one molecule of oxygen to form two molecules of water, with no atoms lost or gained.
Question 8.
1. Write the balanced chemical equation of the following reaction. sodium hydroxide + sulphuric acid → sodium sulphate + water
2. Write the balanced chemical equation of the following reaction.
potassium bicarbonate + sulphuric acid → potassium sulphate + carbon dioxide + water
3. Write the balanced chemical equation of the following reaction. iron + sulphuric acid → ferrous sulphate + hydrogen.
4. Write the balanced chemical equations of the following reactions. chlorine + sulphur dioxide + water → sulphuric acid + hydrogen chloride
5. Write the balanced chemical equation of the following reaction. silver nitrate → silver + nitrogen dioxide + oxygen
6. Write the balanced chemical equation of the following reaction. copper + nitric acid → copper nitrate + nitric oxide + water
7. Write the balanced chemical equation of the following reaction. ammonia + oxygen → nitric oxide + water
8. Write the balanced chemical equation of the following reaction. barium chloride + sulphuric acid → barium sulphate + hydrochloric acid
9. Write the balanced chemical equation of the following reaction. zinc sulphide + oxygen → zinc oxide + sulphur dioxide
10. Write the balanced chemical equation of the following reaction. aluminium carbide + water → aluminium hydroxide + methane
11. Write the balanced chemical equation of the following reaction. iron pyrites(FeS2) + oxygen → ferric oxide + sulphur dioxide
12. Write the balanced chemical equation of the following reaction. Potassium permanganate + hydrochloric acid → potassium chloride + manganese chloride + chlorine + water
13. Write the balanced chemical equation of the following reaction. aluminium sulphate + sodium hydroxide → sodium sulphate + sodium meta aluminate + water.
14. Write the balanced chemical equation of the following reaction. aluminium + sodium hydroxide + water → sodium meta aluminate + hydrogen
15. Write the balanced chemical equation of the following reaction. potassium dichromate + sulphuric acid → potassium sulphate + chromium sulphate + water + oxygen.
16. Write the balanced chemical equation of the following reaction. potassium dichromate + hydrochloric acid → Potassium chloride + chromium chloride + water + chlorine
17. Write the balanced chemical equation of the following reaction. sulphur + nitric acid→ sulphuric acid + nitrogen dioxide + water.
18. Write the balanced chemical equation of the following reaction. sodium chloride + manganese dioxide + sulphuric acid → sodium hydrogen sulphate + manganese sulphate + water + chlorine.
Ans:
- This is a neutralization reaction where an acid and a base react to form salt and water.
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O - Here, the bicarbonate reacts with the acid, releasing carbon dioxide gas.
2KHCO₃ + H₂SO₄ → K₂SO₄ + 2CO₂ + 2H₂O - A reactive metal displaces hydrogen from a dilute acid in this single displacement reaction.
Fe + H₂SO₄ → FeSO₄ + H₂ - Chlorine acts as an oxidizing agent in this reaction with sulphur dioxide.
Cl₂ + SO₂ + 2H₂O → H₂SO₄ + 2HCl - This is a thermal decomposition reaction where a compound breaks down into simpler substances upon heating.
2AgNO₃ → 2Ag + 2NO₂ + O₂ - Copper reacts with moderately concentrated nitric acid, producing nitric oxide.
3Cu + 8HNO₃ → 3Cu(NO₃)₂ + 2NO + 4H₂O - This is a catalytic oxidation reaction, a key step in the industrial production of nitric acid.
4NH₃ + 5O₂ → 4NO + 6H₂O - The formation of a white precipitate of barium sulphate characterizes this double displacement reaction.
BaCl₂ + H₂SO₄ → BaSO₄ + 2HCl - The sulphide ore is roasted in air to convert it into an oxide, releasing sulphur dioxide gas.
2ZnS + 3O₂ → 2ZnO + 2SO₂ - A metallic carbide hydrolyzes with water to produce a hydroxide and a hydrocarbon gas.
Al₄C₃ + 12H₂O → 4Al(OH)₃ + 3CH₄ - The roasting of iron pyrites in air is a key process in the manufacture of sulphuric acid.
4FeS₂ + 11O₂ → 2Fe₂O₃ + 8SO₂ - Potassium permanganate acts as a powerful oxidizing agent in acidic medium, liberating chlorine.
2KMnO₄ + 16HCl → 2KCl + 2MnCl₂ + 5Cl₂ + 8H₂O - Aluminium salts react with bases to form soluble meta-aluminates.
Al₂(SO₄)₃ + 8NaOH → 3Na₂SO₄ + 2NaAlO₂ + 4H₂O - Amphoteric metals like aluminium react with alkalis to produce hydrogen gas.
2Al + 2NaOH + 6H₂O → 2NaAlO₂ + 3H₂ + 4H₂O (or 2Na[Al(OH)₄] + 3H₂) - This is an unusual decomposition reaction for potassium dichromate in the presence of an acid.
2K₂Cr₂O₇ + 8H₂SO₄ → 2K₂SO₄ + 2Cr₂(SO₄)₃ + 8H₂O + 3O₂ - Potassium dichromate oxidizes hydrochloric acid to chlorine gas in this reaction.
K₂Cr₂O₇ + 14HCl → 2KCl + 2CrCl₃ + 7H₂O + 3Cl₂ - Concentrated nitric acid acts as an oxidizing agent to convert sulphur to sulphuric acid.
S + 6HNO₃ → H₂SO₄ + 6NO₂ + 2H₂O - This is a laboratory method for the preparation of chlorine gas.
4NaCl + MnO₂ + 4H₂SO₄ → 4NaHSO₄ + MnSO₄ + 2H₂O + Cl₂
Question 9.
1. Define atomic mass unit.
2. Calculate the molecular mass of the following: Na2SO4.10H2O
Given atomic mass of Cu = 63·5, H = 1, O = 16, C = 12, N = 14, Mg = 24, S = 32
3. Calculate the molecular mass of the following: (NH4)2CO3
Given atomic mass of Cu = 63·5, H = 1, O= 16, C = 12, N = 14, Mg = 24, S = 32
4. Calculate the molecular mass of the following: (NH2)2CO
Given atomic mass of Cu = 63·5, H = 1, O= 16, C = 12, N = 14, Mg = 24, S = 32
5. Calculate the molecular mass of the following: Mg3N2
Given atomic mass of Cu = 63·5, H = 1, O = 16, C = 12, N = 14, Mg = 24, S = 32
Ans:
1. Definition of Atomic Mass Unit
An atomic mass unit (amu) is a standard unit of mass that is used to express the masses of atoms, molecules, and subatomic particles. It is defined as exactly one-twelfth the mass of a single carbon-12 atom. In numerical terms, 1 amu is equal to 1.66053906660 × 10⁻²⁷ kilograms.
2. Calculate the molecular mass of Na₂SO₄·10H₂O
First, it’s helpful to separate the compound into its anhydrous part and the water of crystallization: Na₂SO₄ and 10H₂O.
Mass of Na₂SO₄:
- Sodium (Na): 2 atoms × 23 u = 46 u
- Sulfur (S): 1 atom × 32 u = 32 u
- Oxygen (O): 4 atoms × 16 u = 64 u
- Total for Na₂SO₄ = 46 + 32 + 64 = 142 u
Mass of 10H₂O:
- A single water molecule (H₂O) has a mass of (2 × 1 u) + 16 u = 18 u.
- Therefore, 10 molecules have a mass of 10 × 18 u = 180 u
Total Molecular Mass:
- Mass of Na₂SO₄ + Mass of 10H₂O = 142 u + 180 u = 322 u
Answer: The molecular mass of Na₂SO₄·10H₂O is 322 u.
3. Calculate the molecular mass of (NH₄)₂CO₃
We break down the formula based on the atoms present. The subscript ‘2’ outside the (NH₄) bracket means we have two NH₄ groups.
- Nitrogen (N): 2 atoms × 14 u = 28 u
- Hydrogen (H): 8 atoms × 1 u = 8 u (since (NH₄)₂ gives us 2 × 4 = 8 H atoms)
- Carbon (C): 1 atom × 12 u = 12 u
- Oxygen (O): 3 atoms × 16 u = 48 u
Total Molecular Mass = 28 + 8 + 12 + 48 = 96 u
Answer: The molecular mass of (NH₄)₂CO₃ is 96 u.
4. Calculate the molecular mass of (NH₂)₂CO
This compound is Urea. The formula shows two NH₂ groups attached to a carbonyl (C=O) group.
- Nitrogen (N): 2 atoms × 14 u = 28 u
- Hydrogen (H): 4 atoms × 1 u = 4 u (since (NH₂)₂ gives us 2 × 2 = 4 H atoms)
- Carbon (C): 1 atom × 12 u = 12 u
- Oxygen (O): 1 atom × 16 u = 16 u
Total Molecular Mass = 28 + 4 + 12 + 16 = 60 u
Answer: The molecular mass of (NH₂)₂CO (Urea) is 60 u.
5. Calculate the molecular mass of Mg₃N₂
This is magnesium nitride. The formula is straightforward.
- Magnesium (Mg): 3 atoms × 24 u = 72 u
- Nitrogen (N): 2 atoms × 14 u = 28 u
Total Molecular Mass = 72 + 28 = 100 u
Answer: The molecular mass of Mg₃N₂ is 100 u.
Question 10.
1. Choose the correct answer from the option given below. Modern atomic symbols are based on the method proposed by
- Bohr
- Dalton
- Berzelius
- Alchemist
2. Choose the correct answer from the options given below. The number of carbon atoms in a hydrogen carbonate radical is
- One
- Two
- Three
- Four
3. Choose the correct answer from the options given below. The formula of iron (III) sulphate is ______.
- Fe3SO4
- Fe(SO4)3
- Fe2(SO4)3
- FeSO4
4. Choose the correct answer from the options given below. In water, the hydrogen-to-oxygen mass ratio is
- 1: 8
- 1: 16
- 1: 32
- 1: 64
5. Choose the correct answer from the options given below. The formula of sodium carbonate is Na2CO3 and that of calcium hydrogen carbonate is
- CaHCO3
- Ca(HCO3)2
- Ca2HCO3
- Ca(HCO3)3
Question 11.
1. Correct the following statement: A molecular formula represents an element.
2. Correct the following statement: Molecular formula of water is H2O2
3. Correct the following statement: A molecule of sulphur is monoatomic.
4. Correct the following statement: CO and CO both represent cobalt.
5. Correct the following statement: The formula of iron (lll) oxide is FeO.
Ans:
- Correction: A molecular formula represents a molecule of a compound, which is made of two or more different elements chemically combined. An element, especially in its gaseous form, can also have a molecular formula (like O₂ or S₈), but the statement is misleading. A more accurate way to put it is that a molecular formula shows the exact number and type of atoms in a molecule of a substance.
- Correction: The correct molecular formula of water is H₂O. The formula H₂O₂ represents hydrogen peroxide, which is a different chemical with distinct properties. A water molecule consists of two hydrogen atoms bonded to a single oxygen atom.
- Correction: A molecule of sulphur, under standard conditions, is polyatomic. It exists as a stable ring of eight atoms, giving it the molecular formula S₈. Noble gases like helium or neon are examples of monoatomic molecules.
- Correction: Co is the chemical symbol for the element cobalt. CO is the formula for carbon monoxide, a poisonous gas composed of one carbon atom and one oxygen atom. The distinction between uppercase and lowercase letters is critical in chemical symbols.
- Correction: The formula for iron(III) oxide is Fe₂O₃. The Roman numeral III indicates the iron ion has a +3 charge (Fe³⁺), while the oxide ion has a -2 charge (O²⁻). To create a neutral compound, two iron ions (+6 total charge) balance with three oxygen ions (-6 total charge). FeO is the formula for iron(II) oxide, where the iron has a +2 charge.
Question 12.
1. Calculate the relative molecular masses of the following CHCI3
[At mass : C = 12, H = 1, O = 16, Ci = 35.5, N = 14, Cu = 63.5, S = 32, K = 39, Pt = 195, Ca = 40, P = 31, Mg = 24]
2. Calculate the relative molecular masses of the following (NH4)2 Cr2O7
[At mass : C = 12, H = 1, O = 16, Ci = 35.5, N = 14, Cu = 63.5, S = 32, K = 39, Pt = 195, Ca = 40, P = 31, Mg = 24]
3. Calculate the molecular mass of the following:CuSO4·5H2O
Given atomic mass of Cu = 63·5, H = 1, O = 16, C=12, N = 14, Mg = 24, S = 32
4. Calculate the molecular mass of the following:(NH₄)₂SO₄
[At mass : C = 12, H = 1, O = 16, Ci = 35.5, N = 14, Cu = 63.5, S = 32, K = 39, Pt = 195, Ca = 40, P = 31, Mg = 24]
5. Calculate the molecular mass of the following:
CH3COONa[At mass : C = 12, H = 1, O = 16, Cl = 35.5, N = 14, Cu = 63.5, S = 32, Na = 23, K = 39, Pt = 195, Ca = 40, P = 31, Mg = 24]
6. Calculate the molecular mass of the following:Potassium Chlorate.
At mass : C = 12, H = 1, O = 16, Cl = 35.5, N = 14, Cu = 63.5, S = 32, Na = 23, K = 39, Pt = 195, Ca = 40, P = 31, Mg = 24]
7. Calculate the molecular mass of the following: Ammonium chloroplatinate (NH4)2 PtCl6 [At mass: C = 12, H = 1, O = 16, Cl = 35.5, N = 14, Cu = 63.5, S = 32, K = 39, Pt = 195, Ca = 40, P = 31, Mg = 24]
Ans:
1. Calculate the relative molecular mass of CHCl₃
This compound is chloroform.
- Carbon (C): 1 atom × 12 = 12
- Hydrogen (H): 1 atom × 1 = 1
- Chlorine (Cl): 3 atoms × 35.5 = 106.5
Relative Molecular Mass = 12 + 1 + 106.5 = 119.5
2. Calculate the relative molecular mass of (NH₄)₂Cr₂O₇
This compound is ammonium dichromate.
- Nitrogen (N): 2 atoms × 14 = 28
(Note: The (NH₄)₂ group contains 2 Nitrogen atoms) - Hydrogen (H): 8 atoms × 1 = 8
(Note: The (NH₄)₂ group contains 8 Hydrogen atoms (4 from each NH₄)) - Chromium (Cr): 2 atoms × 52 = 104
(Note: The atomic mass of Chromium (Cr) is a standard 52, which is implied from the formula) - Oxygen (O): 7 atoms × 16 = 112
Relative Molecular Mass = 28 + 8 + 104 + 112 = 252
3. Calculate the molecular mass of CuSO₄·5H₂O
This compound is copper(II) sulfate pentahydrate.
- Copper (Cu): 1 atom × 63.5 = 63.5
- Sulfur (S): 1 atom × 32 = 32
- Oxygen from SO₄: 4 atoms × 16 = 64
- Water of Crystallization (5H₂O):
- Hydrogen: 10 atoms × 1 = 10
- Oxygen: 5 atoms × 16 = 80
Molecular Mass = 63.5 + 32 + 64 + 10 + 80 = 249.5
4. Calculate the molecular mass of (NH₄)₂SO₄
This compound is ammonium sulfate.
- Nitrogen (N): 2 atoms × 14 = 28
- Hydrogen (H): 8 atoms × 1 = 8
- Sulfur (S): 1 atom × 32 = 32
- Oxygen (O): 4 atoms × 16 = 64
Molecular Mass = 28 + 8 + 32 + 64 = 132
5. Calculate the molecular mass of CH₃COONa
This compound is sodium acetate.
- Carbon (C): 2 atoms × 12 = 24
- Hydrogen (H): 3 atoms × 1 = 3
- Oxygen (O): 2 atoms × 16 = 32
- Sodium (Na): 1 atom × 23 = 23
Molecular Mass = 24 + 3 + 32 + 23 = 82
6. Calculate the molecular mass of Potassium Chlorate
The chemical formula for potassium chlorate is KClO₃.
- Potassium (K): 1 atom × 39 = 39
- Chlorine (Cl): 1 atom × 35.5 = 35.5
- Oxygen (O): 3 atoms × 16 = 48
Molecular Mass = 39 + 35.5 + 48 = 122.5
7. Calculate the molecular mass of Ammonium chloroplatinate (NH₄)₂PtCl₆
- Nitrogen (N): 2 atoms × 14 = 28
- Hydrogen (H): 8 atoms × 1 = 8
- Platinum (Pt): 1 atom × 195 = 195
- Chlorine (Cl): 6 atoms × 35.5 = 213
Molecular Mass = 28 + 8 + 195 + 213 = 444
Question 13.
1.Give the empirical formula of : Benzene C6H6
2. Give the empirical formula of : Glucose C6H12O6 .
3. Give the empirical formula of : Acetylene C2H2
4. Give the empirical formula of : Acetic Acid CH3COOH.
Ans:
A molecular formula shows the exact number of each type of atom in a molecule. An empirical formula, in contrast, shows the simplest whole-number ratio of these atoms. It represents the fundamental proportion of elements within a compound. Here are several common examples that illustrate this concept.
1. Benzene (C₆H₆)
- Molecular Formula: C₆H₆
- Empirical Formula: CH
- Breakdown: In benzene, the ratio of carbon (C) to hydrogen (H) atoms is 6:6. This ratio can be simplified by dividing both numbers by 6, which gives a reduced ratio of 1:1. Therefore, the empirical formula is CH.
2. Glucose (C₆H₁₂O₆)
- Molecular Formula: C₆H₁₂O₆
- Empirical Formula: CH₂O
- Breakdown: The molecular formula shows 6 carbon, 12 hydrogen, and 6 oxygen atoms. The ratio is 6:12:6. Dividing all three numbers by their greatest common divisor, which is 6, simplifies the ratio to 1:2:1. This gives us the empirical formula CH₂O. Interestingly, this is the same empirical formula as formaldehyde.
3. Acetylene (C₂H₂)
- Molecular Formula: C₂H₂
- Empirical Formula: CH
- Breakdown: Acetylene contains 2 carbon atoms and 2 hydrogen atoms, a ratio of 2:2. When we divide both subscripts by 2, the ratio simplifies to 1:1. This results in an empirical formula of CH, identical to that of benzene, despite the two molecules being very different chemically.
4. Acetic Acid (C₂H₄O₂)
- Molecular Formula: C₂H₄O₂
- Empirical Formula: CH₂O
- Breakdown: The molecular formula indicates atoms in a 2:4:2 ratio for carbon, hydrogen, and oxygen, respectively. The greatest common divisor here is 2. Dividing all subscripts by 2 provides the simplest ratio of 1:2:1, leading to the empirical formula CH₂O. This shows that acetic acid shares the same basic elemental ratio as glucose and many sugars.
Question 14.
Find the percentage of mass water in Epsom salt MgSO4.7H2O.
Ans:
Step 1: Determine the Molar Mass
To begin, the molar mass of the compound MgSO₄·7H₂O is calculated by summing the atomic masses of all atoms in the formula.
- Magnesium (Mg): 24.305 g/mol
- Sulfur (S): 32.06 g/mol
- Oxygen (in SO₄): 4 × 16.00 g/mol = 64.00 g/mol
Mass of MgSO₄ unit:
24.305 + 32.06 + 64.00 = 120.365 g/mol
For the water molecules:
- Mass of one H₂O molecule: (2 × 1.008) + 16.00 = 18.016 g/mol
- Mass of seven H₂O molecules: 7 × 18.016 = 126.112 g/mol
Total molar mass of the hydrate:
120.365 g/mol + 126.112 g/mol = 246.477 g/mol
Step 2: Identify the Mass of Water
In one mole of MgSO₄·7H₂O, the total mass contribution from water is 126.112 g.
Step 3: Compute the Percentage of Water by Mass
Using the formula:
(Mass of Water / Total Mass of Hydrate) × 100%
Percentage of Water = (126.112 / 246.477) × 100%
Step 4: Final Calculation and Result
126.112 ÷ 246.477 ≈ 0.5117
0.5117 × 100% = 51.17%
Thus, the percentage of water by mass in Epsom salt (MgSO₄·7H₂O) is 51.17%.
Question 15.
1. Calculate the percentage of phosphorus in calcium hydrogen phosphate Ca(H2PO4)2 .
2. Calculate the percentage of phosphorus in calcium phosphate Ca3(PO4)2.
Ans:
1. Percentage of Phosphorus in Calcium Hydrogen Phosphate, Ca(H₂PO₄)₂
Step 1: Calculate the molar mass of Ca(H₂PO₄)₂.
- Calcium (Ca): 40.08 g/mol
- Hydrogen (H): 1.008 g/mol × 4 = 4.032 g/mol (There are 4 H atoms per (H₂PO₄) group, and two such groups, so 4 × 2 = 8 H atoms total. We will calculate by group to avoid error).
- Phosphorus (P): 30.97 g/mol × 2 = 61.94 g/mol
- Oxygen (O): 64.00 g/mol × 8 = 128.00 g/mol (There are 8 O atoms per (H₂PO₄) group, and two such groups, so 8 × 2 = 16 O atoms total).
Let’s calculate the mass of one (H₂PO₄) group first:
(2 × 1.008) + 30.97 + (4 × 16.00) = 2.016 + 30.97 + 64.00 = 96.986 g/mol
Since there are two of these groups: 96.986 × 2 = 193.972 g/mol
Now, add the mass of Calcium:
Molar Mass = 40.08 + 193.972 = 234.052 g/mol
Step 2: Calculate the total mass of Phosphorus in one mole.
The formula Ca(H₂PO₄)₂ contains 2 atoms of Phosphorus.
Total mass of P = 2 × 30.97 g/mol = 61.94 g/mol
Step 3: Calculate the percentage composition.
% Phosphorus = (Total mass of P / Molar Mass of Compound) × 100%
% P = (61.94 / 234.052) × 100%
% P = 26.47%
Answer: The percentage of phosphorus in calcium hydrogen phosphate is 26.47%.
2. Percentage of Phosphorus in Calcium Phosphate, Ca₃(PO₄)₂
Step 1: Calculate the molar mass of Ca₃(PO₄)₂.
- Calcium (Ca): 40.08 g/mol × 3 = 120.24 g/mol
- Phosphorus (P): 30.97 g/mol × 2 = 61.94 g/mol
- Oxygen (O): 16.00 g/mol × 8 = 128.00 g/mol
Molar Mass = 120.24 + 61.94 + 128.00 = 310.18 g/mol
Step 2: Calculate the total mass of Phosphorus in one mole.
The formula Ca₃(PO₄)₂ contains 2 atoms of Phosphorus.
Total mass of P = 2 × 30.97 g/mol = 61.94 g/mol
Step 3: Calculate the percentage composition.
% Phosphorus = (Total mass of P / Molar Mass of Compound) × 100%
% P = (61.94 / 310.18) × 100%
% P = 19.97%
Answer: The percentage of phosphorus in calcium phosphate is 19.97%.
Question 16.
Calculate the percentage composition of each element in Potassium chlorate KCIO3.
Ans:
Step 1: Identifying Atomic Masses of the Elements
We begin by noting the average atomic masses for the relevant elements:
- Potassium (K): 39.10 u
- Chlorine (Cl): 35.45 u
- Oxygen (O): 16.00 u
Step 2: Calculating the Molar Mass of Potassium Chlorate
The chemical formula KClO₃ tells us that each unit contains one potassium atom, one chlorine atom, and three oxygen atoms. Thus, the molar mass is the sum of the masses of these atoms.
- Potassium: 1 × 39.10 g/mol = 39.10 g/mol
- Chlorine: 1 × 35.45 g/mol = 35.45 g/mol
- Oxygen: 3 × 16.00 g/mol = 48.00 g/mol
Adding these values gives the total molar mass:
39.10 g/mol + 35.45 g/mol + 48.00 g/mol = 122.55 g/mol
Step 3: Determining the Percentage of Each Element
Using the molar mass obtained, we calculate the mass percentage for each element.
- Potassium (K):
Mass contributed = 39.10 g/mol
Percentage = (39.10 ÷ 122.55) × 100 = 31.91% - Chlorine (Cl):
Mass contributed = 35.45 g/mol
Percentage = (35.45 ÷ 122.55) × 100 = 28.93% - Oxygen (O):
Mass contributed = 48.00 g/mol
Percentage = (48.00 ÷ 122.55) × 100 = 39.16%
Step 4: Verifying the Results
To check the accuracy of the calculations, we add the individual percentages:
31.91% + 28.93% + 39.16% = 100.00%
The total confirms that the values are consistent.
Final Results
The percentage composition by mass of potassium chlorate (KClO₃) is:
- Potassium (K): 31.91%
- Chlorine (Cl): 28.93%
- Oxygen (O): 39.16%
Question 17.
Urea is a very important nitrogenous fertilizer. Its formula is CON2H4. Calculate the percentage of carbon in urea. (C = 12, O = 16, N = 14 and H = 1)
Ans:
To find the percentage of carbon in urea (CON₂H₄), we must first determine its total molecular mass. The formula CON₂H₄ indicates the molecule contains one Carbon atom, one Oxygen atom, two Nitrogen atoms, and four Hydrogen atoms.
Using the provided atomic masses:
- Mass from Carbon (C) = 1 × 12 = 12 u
- Mass from Oxygen (O) = 1 × 16 = 16 u
- Mass from Nitrogen (N) = 2 × 14 = 28 u
- Mass from Hydrogen (H) = 4 × 1 = 4 u
Adding these together gives the total molecular mass of urea:
12 + 16 + 28 + 4 = 60 u.
formula:
(Mass of the element in the molecule / Total molecular mass) × 100.
Therefore, the percentage of carbon is:
(12 / 60) × 100 = 0.2 × 100 = 20%.
Thus, the final result is that urea contains 20% carbon by mass.