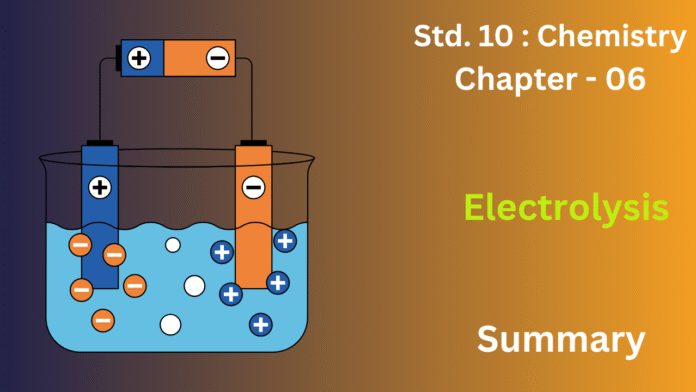
1. Basic Concept:
Electrolysis is a chemical process where a substance (an electrolyte) is decomposed into its constituent elements by passing an electric current through it in its molten state or in an aqueous solution. It requires a battery, two electrodes (anode and cathode), and an electrolyte.
2. Key Definitions:
Electrolyte: A compound (like acid, base, or salt) which in molten or aqueous state conducts electricity and gets decomposed.
Electrodes: Conductors that carry current into and out of the electrolyte.
Anode: The positive electrode connected to the battery’s positive terminal. Oxidation occurs here.
Cathode: The negative electrode connected to the battery’s negative terminal. Reduction occurs here.
3. The Process (How it Works):
The battery provides energy to pull ions apart. Positive ions (cations) are attracted to the cathode, where they gain electrons (reduction). Negative ions (anions) are attracted to the anode, where they lose electrons (oxidation).
4. Core Principles:
Preferential Discharge of Ions: At each electrode, if multiple ions are present, one is selectively discharged based on:
Position in the Electrochemical Series: Ions lower in the series are discharged more readily.
Concentration of Ions: A highly concentrated ion may be discharged even if it’s higher in the series.
Nature of the Electrode: Inert electrodes (like graphite) don’t react, while reactive electrodes (like copper) can dissolve.
5. Important Applications:
Electroplating: Coating a cheap metal object with a layer of a superior metal (like chromium or silver) using electrolysis.
Purification of Metals: Impure copper is made the anode and a pure copper strip is the cathode. Pure copper deposits on the cathode.
Extraction of Reactive Metals: Highly reactive metals like sodium, aluminum, and potassium are extracted from their molten ores using electrolysis.
In short, electrolysis uses electricity to break down compounds and is crucial for processes like electroplating and metal extraction.
INTEXT – QUESTION – 1
1) FILL IN THE BLANKS:
(a) Powdered sodium chloride (common salt) does not conduct an electric current, but it does so when ……………. or when ……………..
(b) Molten lead bromide conducts electricity .It is called an …………….. It is composed of lead …………….and bromide …………….. The lead ions are ……………. charged and are called …………….. The bromide …………….are …………….charged and are called …………….. (c) Substances which conduct electricity in the solid state are generally ……………..
(d) The electron releasing tendency of zinc is ……………. than that of copper.
(e) A solution of HCl gas in water conducts electricity because ……………., but a solution of HCl gas in toluene does not conduct an electric current because …………….
Ans: (a) Powdered sodium chloride (common salt) does not conduct an electric current, but it does so when dissolved in water or when melted.
(b) Molten lead bromide conducts electricity .It is called an electrolyte. It is composed of lead ions and bromide ions. The lead ions are positively charged and are called cations. The bromide ions are negatively charged and are called anions.
(c) Substances which conduct electricity in the solid state are generally metals.
(d) The electron releasing tendency of zinc is more than that of copper.
(e) A solution of HCl gas in water conducts electricity because it ionizes, but a solution of HCl gas in toluene does not conduct an electric current because it does not ionize in toluene.
2) Define the following terms:
(a) Electrolysis,
(b) Non-electrolyte,
(c) Cation and anion,
(d) Weak electrolyte,
Ans: (a) Electrolysis
A process using direct current (DC) to decompose an ionic compound (molten or dissolved) into its elements via a non-spontaneous reaction.
(b) Non-electrolyte
A substance that does not conduct electricity in any state (e.g., solid, molten, aqueous) due to absence of ions. Examples: sugar, urea.
(c) Cation and Anion
Cation: Positively charged ion; migrates to the cathode (negative electrode).
Anion: Negatively charged ion; migrates to the anode (positive electrode).
(d) Weak Electrolyte
A substance that partially ionizes in solution, showing low electrical conductivity. Examples: acetic acid, ammonia.
3) What is the difference between:
(a) Modern explanation and Arrhenius explanation for the theory of electrolysis:
(b) electrolytic dissociation and ionization :
(c) A cation and an anion,
Ans: (a) Modern Explanation vs. Arrhenius Explanation for Electrolysis
Arrhenius Explanation (1887): Proposed that electrolytes (acids, bases, salts) dissociate into ions simply by being dissolved in water. He believed the water itself was responsible for splitting the compound.
Modern Explanation: Expands on Arrhenius’s work. It states that ionic compounds already exist as ions in their solid state (in a lattice). When dissolved or melted, this lattice breaks down, setting the pre-existing ions free to move. For covalent compounds (like HCl), it agrees that they ionize upon dissolution.
(b) Electrolytic Dissociation vs. Ionization
Electrolytic Dissociation: This term refers to the process where an ionic compound (e.g., NaCl) splits or separates into its constituent ions when dissolved in a solvent like water or melted. The ions pre-exist in the solid.
Ionization: This term refers to the process where a neutral covalent molecule (e.g., HCl) gains or loses electrons to form ions when it reacts with a solvent like water. New ions are created during this process.
(c) Cation vs. Anion
Cation: An ion with a positive charge. It is attracted to the cathode (negative electrode) during electrolysis. Cations are formed when an atom loses electrons (e.g., Na⁺, Ca²⁺).
Anion: An ion with a negative charge. It is attracted to the anode (positive electrode) during electrolysis. Anions are formed when an atom gains electrons (e.g., Cl⁻, O²⁻).
4) Name:
(a) a salt which is a weak electrolyte
(b) a base which is a weak electrolyte,
(c) an inert electrode and an active electrode,
(d) a positively charged non-metallic ion,
(e) the electrode at which reduction occurs,
(f) a non-metallic element which is a conductor of electricity.
Ans: (a) Sodium carbonate
(b) NH4OH
(c) An inert electrode: graphite and Active electrode: silver
(d) H+
(e) Electrode is cathode
(f) Graphite
5) Electrolysis is a redox process. Explain.
Ans: Electrolysis is a redox process because it involves both oxidation and reduction reactions occurring simultaneously.
Oxidation (loss of electrons) happens at the anode (positive electrode). For example, chloride ions (Cl⁻) can lose electrons to form chlorine gas (Cl₂).
Reduction (gain of electrons) happens at the cathode (negative electrode). For example, sodium ions (Na⁺) can gain electrons to form sodium metal (Na).
These two half-reactions are forced to occur by an external electrical power source, which supplies the energy needed to drive this non-spontaneous redox reaction.
INTEXT – QUESTION – 2
1) Name two substances in each case:
(a) Contain only molecules,
(b) Contain only ions,
(c) Contain ions as well as molecules.
Ans: (a) Glucose, Kerosene
(b) NaCl and NaOH
(c) CH3COOH and NH4OH
2) Explain the following:
(a) A solution of cane sugar does not conduct electricity, but a solution of solution of sodium chloride is a good conductor,
(b) Hydrochloric acid is a good conductor of electricity,
(c) During the electrolysis of an aqueous solution of NaCI, hydrogen ion is reduced at the cathode and not the sodium ion though both Na+ and H+ ions are present in the solution.
Ans:
(a) Cane Sugar vs. Sodium Chloride Solution
Cane sugar (sucrose) is a covalent compound. When dissolved, it does not dissociate into ions. It exists as neutral molecules. Since there are no charged particles (ions) to carry the electric current, the solution does not conduct electricity.
Sodium chloride (NaCl) is an ionic compound. When dissolved in water, it dissociates into mobile Na⁺ and Cl⁻ ions. These free-moving ions act as charge carriers, allowing the solution to conduct electricity.
(b) Hydrochloric Acid as a Good Conductor
Hydrochloric acid (HCl) is a strong acid. When dissolved in water, it completely ionizes into H⁺ (or H₃O⁺) ions and Cl⁻ ions.
The high concentration of these free-moving ions in the solution makes hydrochloric acid a very good conductor of electricity.
(c) Reduction at the Cathode during NaCl Electrolysis
During the electrolysis of aqueous sodium chloride, both Na⁺ ions and H⁺ ions (from water) are present near the cathode.
The H⁺ ions from water are more easily reduced (gain electrons) than Na⁺ ions. This is because hydrogen has a much higher discharge potential (or is less electropositive) compared to sodium.
As a result, hydrogen gas is liberated at the cathode due to the reduction of H⁺ ions, while the Na⁺ ions remain in the solution.
Reaction: 2H⁺ + 2e⁻ → H₂(g)
3) (a) Among Zn and Cu, which would occur more readily in nature as metal and which as ion?
(b) Why cannot we store AgNO3 solution in copper vessels?
(c) Out of Cu and Ag, which is more active?
Ans:
(a) Occurrence in Nature
Zinc is more reactive, so it commonly forms compounds like Zn²⁺ ions.
Copper is less reactive, so it is often found as native metal.
(b) Storing AgNO₃ in Copper Vessel
Copper is more reactive than silver.
Copper displaces silver from AgNO₃ solution:
Cu + 2AgNO₃ → Cu(NO₃)₂ + 2Ag
Silver deposits and the solution reacts, so storage is not possible.
(c) More Reactive Metal
Copper is more reactive than silver.
4) (a) How would you change a metal like Cu into its ions?
(b) how would you change Cu2+ ion to Cu?
Ans:
(a) Changing Copper Metal (Cu) into its Ions
Copper metal can be changed into its ions (Cu²⁺) through a chemical reaction where it loses electrons. This process is called oxidation.A common way is to dissolve it in a strong acid like nitric acid or in an acid with an oxidizing agent. For example, it reacts with sulfuric acid in the presence of air (oxygen).
Reaction:
2Cu + O₂ + 2H₂SO₄ → 2CuSO₄ + 2H₂O
Here, Cu is oxidized to Cu²⁺ ions.
(b) Changing Cu²⁺ ion to Copper Metal (Cu)
Cu²⁺ ions can be changed back into copper metal by gaining electrons. This process is called reduction.A simple method is electrolysis of a copper salt solution, where an electric current forces the Cu²⁺ ions to accept electrons and deposit as neutral copper metal on the cathode.
Reaction at Cathode:
Cu²⁺ + 2e⁻ → Cu
5) A solution of caustic soda (NaOH) in water or when fused, conducts an electric current. What is the similarity in these two cases?
Ans: In both cases, the electric current is carried by the movement of ions.In an aqueous NaOH solution, the NaOH dissociates into mobile Na⁺ and OH⁻ ions in the water.In fused (molten) NaOH, the high temperature breaks the ionic bonds, creating a liquid of free-moving Na⁺ and OH⁻ ions.The similarity is that in both states, the presence of these free-moving ions allows for the conduction of electricity.
6) During electrolysis of an aqueous solution of sulphuric acid between platinum electrodes, two types of anions migrate towards the anode but only one of themis discharged:
(a) Name the two anions,
(b) Name the main product of the discharge of anion at the anode and write the anode reaction,
(c) Name the product at the cathode and write the reaction.
(d) How do you notice any change in colour? State why?
(e) Why is this electrolysis considered as an example of catalysis?
Ans: (a) Name the two anions
The two anions migrating towards the anode are sulphate ions (SO₄²⁻) and hydroxide ions (OH⁻).
(b) Name the main product and write the anode reaction
Main Product: Oxygen gas (O₂).
Anode Reaction: 4OH⁻(aq) → 2H₂O(l) + O₂(g) + 4e⁻
(c) Name the product at the cathode and write the reaction
Product at Cathode: Hydrogen gas (H₂).
Cathode Reaction: 4H⁺(aq) + 4e⁻ → 2H₂(g)
(d) Change in colour and reason
Change: The colour of the solution does not change. It remains colourless.
Reason: The electrolysis decomposes water (H₂O) into hydrogen and oxygen gas. The concentration of sulphuric acid remains the same, so there is no change in the intensity of the colourless solution.
(e) Why is it an example of catalysis?
This electrolysis is considered an example of catalysis because sulphuric acid acts as a catalyst. It increases the conductivity of water by providing H⁺ and SO₄²⁻ ions, facilitating the decomposition of water into H₂ and O₂. The acid itself is not consumed in the overall reaction.
Overall Reaction: 2H₂O(l) → 2H₂(g) + O₂(g)
7) An electrolytic cell is set up using two platinum electrodes and an aqueous solution of copper (II) sulphate,
(a)draw a labelled diagram of the electrolytic cell,
(b)Name the ions present in the cell,
(c)Name the ions migrating towards the anode,
(d)Name the ions migrating towards the cathode,
(e)Name the ions which will not be discharged at electrodes during electrolysis, (f)Write the reaction at the cathode,
(g)Write the reaction at the anode,
(h)Name the spectator ion in the solution.
Ans: (a) Labelled Diagram of the Electrolytic Cell
A simple labelled diagram would show:
A container with an Aqueous Solution of CuSO₄.
Two Platinum Electrodes dipped into the solution.
The electrode connected to the positive terminal of a battery is labelled Anode.
The electrode connected to the negative terminal of the battery is labelled Cathode.
A Battery (DC Source) connected to the electrodes.
(b) Ions present in the cell
Cu²⁺, H⁺, SO₄²⁻, OH⁻
(c) Ions migrating towards the anode
SO₄²⁻ and OH⁻
(d) Ions migrating towards the cathode
Cu²⁺ and H⁺
(e) Ions which will not be discharged
SO₄²⁻
(f) Reaction at the cathode
Cu²⁺(aq) + 2e⁻ → Cu(s)
(g) Reaction at the anode
2H₂O(l) → O₂(g) + 4H⁺(aq) + 4e⁻
or 4OH⁻(aq) → O₂(g) + 2H₂O(l) + 4e⁻
(h) Spectator ion in the solution
SO₄²⁻
8) State the electrode reaction at the anode during electrolysis of:
(a) very dilute sulphuric acid,
(b) Aqueous copper sulphate solution
(c) sodium chloride solution,
(d) Fused lead bromide,
(e) magnesium chloride (molten).
Ans: (a) Very dilute sulphuric acid
Reaction: 2H₂O(l) → O₂(g) + 4H⁺(aq) + 4e⁻
Explanation: In very dilute solutions, hydroxide ions (OH⁻) from water are preferentially discharged over sulphate ions, producing oxygen gas.
(b) Aqueous copper sulphate solution
Reaction: 2H₂O(l) → O₂(g) + 4H⁺(aq) + 4e⁻
Explanation: Again, for aqueous solutions using inert electrodes, water molecules are more easily oxidized than sulphate ions, leading to the release of oxygen gas.
(c) Sodium chloride solution (Brine)
Reaction: 2Cl⁻(aq) → Cl₂(g) + 2e⁻
Explanation: In concentrated chloride solutions, chloride ions (Cl⁻) are discharged in preference to hydroxide ions, producing chlorine gas.
(d) Fused lead bromide
Reaction: 2Br⁻(l) → Br₂(g) + 2e⁻
Explanation: In the molten state, the only negative ions present are bromide ions (Br⁻), which are oxidized to form bromine vapor.
(e) Magnesium chloride (molten)
Reaction: 2Cl⁻(l) → Cl₂(g) + 2e⁻
Explanation: Similar to lead bromide, in the molten state, chloride ions (Cl⁻) are the only negative ions available to be discharged, forming chlorine gas.
9) Choosing only words from the following list, write down the appropriate words to fill in the blanks (a) to (e) below: anions, anode, cathode, cations, electrode, electrolyte, nickel, voltameter. The electroplating of an article with nickel requires an
(a)_______which must be a solution containing
(b)____ions. The article to be plated is placed as the
(c)____of the cell in which the plating is carried out. The
(d)____of the cell is made from pure nickel. The ions that are attracted to the negative electrode and discharged are called
(e)________
Ans: (a) Electrolyte
(b) Nickel
(c) Cathode
(d) Anode
(e) Cations
INTEXT – QUESTION – 3
1) Give reasons for the following:
(a) Electrolysis of molten lead bromide is considered to be a reaction in which oxidation and reduction go side by side, i.e., a redox reaction.
(b) The blue colour of aqueous copper sulphate fades when it is electrolysed using platinum electrodes.
(c) Lead bromide undergoes electrolytic dissociation in the molten state but is a non- electrolyte in the solid state.
(d) Aluminium is extracted from its oxide by electrolytic reduction and not by convectional reducing agents.
(e) The ratio of hydrogen and oxygen formed at the cathode and anode is 2: 1 by volume.
(f) In the electrolysis of acidified water, dilute sulphuric acid is preferred to dilute nitric acid for acidification.
(g) Ammonia is unionized in the gaseous state but in the aqueous solution, it is a weak electrolyte.
(h) A graphite anode is preferred to other inert electrodes during electrolysis of fused lead bromide.
(i) for electroplating with silver, silver nitrate is not used as electrolyte.
(j) carbon tetrachloride is a liquid but does not conduct electricity.
Ans: (a) During electrolysis of molten PbBr₂, lead ions are reduced at the cathode (Pb²⁺ → Pb), and bromide ions are oxidized at the anode (2Br⁻ → Br₂). Since oxidation and reduction occur simultaneously, it is a redox reaction.
(b) The blue colour, due to Cu²⁺ ions, fades because these ions are discharged at the cathode (depositing as copper metal), reducing their concentration in the solution.
(c) In the molten state, ions are free to move and conduct electricity. In the solid state, ions are fixed in position and cannot move, so it does not conduct.
(d) Aluminium is too reactive and has a strong affinity for oxygen. It cannot be reduced by common reducing agents like carbon; a stronger reducing agent (via electrolysis) is required.
(e) Acidified water decomposes as 2H₂O → 2H₂ + O₂. The equation shows that for every 2 volumes of hydrogen gas formed, 1 volume of oxygen gas is formed.
(f) Dilute sulphuric acid provides H⁺ and SO₄²⁻ ions which do not interfere with the electrolysis products. Nitric acid can undergo side reactions or produce different gases at the electrodes.
(g) In gaseous state, ammonia exists as NH₃ molecules. In aqueous solution, it reacts with water to form NH₄OH, which partially dissociates into NH₄⁺ and OH⁻ ions, making it a weak electrolyte.
(h) Graphite (carbon) is inert and does not react with bromine vapour produced at the anode, unlike many metals which would corrode.
(i) Silver nitrate solution is not used because the silver ions deposit too rapidly, forming a loose, non-adherent, and coarse layer of silver. Complex cyanide electrolytes are used for a smooth, even coating.
(j) Carbon tetrachloride is a covalent compound. It does not form free ions in the liquid state, so it cannot conduct electricity.
2) Classify the following substance under three headings:
(a) strong electrolytes
(b) Weak electrolytes
(c) Non electrolytes.
Acetic acid, ammonium chloride, ammonium hydroxide, carbon tetrachloride, dilute hydrochloric acid, sodium acetate, dilute sulphuric acid.
Ans: (a) Strong electrolyte: Dilute hydrochloric acid, dilute sulphuric acid, ammonium chloride, sodium acetate
(b) Weak electrolyte: Acetic acid, ammonium hydroxide
(c) Non-electrolyte: Carbon tetrachloride
3) Write down the words or phrases from the brackets that will correctly fill in the blanks in the following sentences:
(a) Pure water consists entirely of ________________ (ions/ molecules).
(b) We can expect that pure water ________________ (will / will not) normally conduct electricity.
Ans: (a) Molecules
(b) Will not
4) To carry out the so-called “electrolysis of water”. Sulphuric acid is added to water. How does the addition of sulpuric acid produce a conducting solution?
Ans: The addition of sulphuric acid to water produces a conducting solution because of ion formation.Pure water is a very poor conductor of electricity as it has very few ions. When sulphuric acid (H₂SO₄) is added, it dissociates (splits apart) in water to produce mobile ions:
H₂SO₄ → 2H⁺ + SO₄²⁻
These positively charged hydrogen ions (H⁺) and negatively charged sulphate ions (SO₄²⁻) are free to move throughout the solution. This movement of charged particles allows the solution to conduct electricity, enabling the electrolysis of water to proceed.
5) Copy and complete the following table which refers to two practical applications of electrolysis
Ans:
| Anode | Electrolyte | Cathode | |
| Silver plating of a spoon | Plate of pure clean | Solution of potassium argentocyanide | Article to be electroplated |
| Purification of copper | Impure copper | Solution of copper sulphate and dilute sulphuric acid | Thin strip of pure copper |
6) Complete the sentence by choosing correct words given in brackets. Electrolysis is the passage of ___________________ (electricity / electrons) through a liquid or a solution accompanied by a ___________ (Physical / chemical) change.
Ans: Electricity, Chemical
Question 2004: Element X is a metal with a valency 2. Element Y is a non-metal with a valency 3.
(a) Write equations to show how x and y form ions?
(b) If Y is a diatomic gas, write the equation for the direct combination of X and Y to form a compound,
(c) Write two applications of electrolysis in which the anode diminishes in mass,
(d) If the compound formed between X and Y is melted and an electric current passes through the molten compound, the element X will be obtained at the ………….. and Y at the ……………….. of the electrolytic cell. (Provide the missing words)
Ans: (a) Formation of ions
X (metal): Loses 2 electrons to form a X²⁺ cation.
Y (non-metal): Gains 3 electrons to form a Y³⁻ anion.
(b) Direct combination
The balanced chemical equation is:
3X + Y₂ → X₃Y₂
(c) Two applications where anode mass decreases
Electroplating: The anode is made of the pure metal to be plated, which dissolves into the solution.
Electrorefining: In copper refining, the impure copper anode dissolves, depositing pure copper at the cathode.
(d) Electrolysis of molten compound
At Cathode: Metal X is obtained.
At Anode: Non-metal Y is obtained.
Question 2004(2): (a) What kind of particles will be found in a liquid compound which is a non – electrolyte?
(b) If HX is a weak acid, what particles will be present in its dilute solution apart from those of water?
(c) Cations are formed by …………….. (loss/gain) of electrons and anions are formed by ………………… (a) (loss / gain) of electrons. (Choose the correct word to fill in the blanks).
(d) What ions must be present in a solution used for electroplating a particular metal?
(e) Explain how electrolysis is an example of redox reaction.
Ans:
(a) True – Non-electrolytes contain only neutral molecules in liquid form.
(b) True – Weak acids partially dissociate into H⁺ and X⁻ ions, so HX molecules and ions coexist.
(c) True – Cations form by electron loss; anions form by electron gain.
(d)Electroplating requires ions of the same metal to be deposited from the solution.
(e)Electrolysis involves simultaneous oxidation at the anode and reduction at the cathode, making it a redox reaction.
Question 2005: 1. Explain why:
(a) Cu, though a good conductor of electricity is a non electrolyte,
(b) Solid sodium chloride does not allow electricity to pass through?
Ans: (a) Copper (a metal): It conducts electricity by the flow of free electrons. This is a physical process where electrons move through the solid metal without causing any chemical change to the copper itself.
Electrolyte: A substance that conducts electricity in its molten state or aqueous solution by the movement of ions. This process always involves a chemical decomposition (electrolysis).
Conclusion: Since copper conducts via electrons and without chemical change, it is classified as a metallic conductor, not an electrolyte.
(b) Why Solid Sodium Chloride does not conduct electricity
In its solid crystal state, sodium chloride (NaCl) consists of ions (Na⁺ and Cl⁻) held in fixed positions by strong electrostatic forces of attraction.
Because the ions are locked in place and cannot move freely, there are no charge carriers available to conduct an electric current through the solid.
Note: When melted or dissolved in water, these ions become free to move and can then conduct electricity.
Question 2005(2): Name the gas released at cathode when acidulated water is electrolyzed.
Ans: When acid is added to water, it provides hydrogen ions (H⁺).
During electrolysis, the negative cathode attracts these positive ions.
Each H⁺ ion gains an electron at the cathode.
Pairs of these hydrogen atoms then combine to form hydrogen gas (H₂), which is seen as bubbles.
Question 2006: Copper sulphate solution is electrolyzed using a platinum anode.
(a) Study the diagram given alongside and answer the following questions:
(i) Give the name of the electrodes A and B.
(ii) Which electrode is the oxidizing electrode?
(b) A strip of copper is placed in four different colourless salt solutions. They are KNO3, AgNO3, Zn(NO3)2, Ca(NO3)2. Which one of the solutions will finally turn blue?
Ans:(a)
(i) A → Cathode (connected to negative terminal)
B → Anode (connected to positive terminal)
(ii) B (Anode) is the oxidizing electrode.
(b)Only AgNO₃ solution will turn blue.
Reason: Copper displaces silver from AgNO₃, forming Cu²⁺ ions which give a blue colour to the solution.
Question 2007: Choose A, B, C or D to match the descriptions (i) to (v) below. Some alphabets may be repeated. A. non-electrolyte B. strong electrolyte C. weak electrolyte D. metallic conductor
(i) Molten ionic compound,
(ii) carbon tetrachloride,
(iii) An aluminium wire,
(iv) A solution containing solvent molecules, solute molecules and ions formed by the dissociation of solute molecules.
(v) A sugar solution with sugar molecules and water molecules.
Ans: (i) B – strong electrolyte
(ii) A – non-electrolyte
(iii) D – metallic conductor
(iv) C – weak electrolyte
(v) A – non-electrolyte
Question 2008:
(a) Here is an electrode reaction: Cu ⟶ Cu2+ + 2e- At which electrode (anode or cathode) would such a reaction take place? Is this an example of oxidation or reduction?
(b) A solution contains magnesium ions (Mg2+), iron (II) ions (Fe2+) and copper ions (Cu2+). On passing an electric current through this solution, which ions will be the first to be discharged at the cathode? Write the equation for the cathode reaction.
(c) Why does carbon tetrachloride, which is a liquid, take place?
Ans:
(a)This reaction occurs at the anode.
It is an example of oxidation.
(b)Cu²⁺ ions will be discharged first at the cathode.
Cathode reaction:
Cu2++2e−⟶CuCu 2++2e − ⟶Cu
(c)Carbon tetrachloride is a liquid but does not conduct electricity because it consists of molecules with no free ions or electrons to carry the current.
Question 2008(2): During the electrolysis of molten lead bromide, which of the following takes place?
A. Bromine is released at the cathode,
B. Lead is deposited at the anode,
C. Bromine ions gain electrons,
D. Lead is deposited at the cathode.
Ans: During electrolysis of molten lead bromide (PbBr₂):
At the cathode: Pb²⁺ ions gain electrons → Lead metal is deposited.
At the anode: Br⁻ ions lose electrons → Bromine gas is released.
Thus, the correct option is:
D. Lead is deposited at the cathode.