Chemical bonding is how atoms attach to each other to form molecules and compounds. Atoms do this to become more stable, typically by achieving a full outer shell of electrons (8, or 2 for small atoms like Helium), known as the octet rule.
1. Why Atoms Bond
Atoms bond to gain stability. They complete their outer electron shell by losing, gaining, or sharing electrons.
2. Main Types of Bonds
a) Ionic Bond
Formed between a metal and a non-metal.
Involves the complete transfer of electrons.
The metal becomes a positive ion (cation), the non-metal becomes a negative ion (anion).
These ions attract each other strongly.
Example: Sodium Chloride (NaCl).
b) Covalent Bond
Formed between two non-metal atoms.
Involves the sharing of electron pairs.
Each shared pair is one covalent bond.
Examples: Hydrogen gas (H₂), Water (H₂O).
c) Coordinate Bond
A special covalent bond where one atom donates both shared electrons.
Identical to a covalent bond once formed.
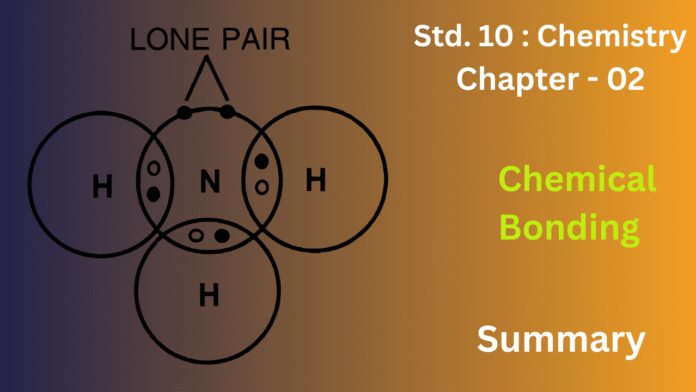
Example: Ammonium ion (NH₄⁺).
3. Key Structures
Crystal Lattice: The strong, orderly 3D arrangement of ions in a solid ionic compound.
4. Properties of Compounds
Ionic Compounds (e.g., NaCl)
High melting and boiling points.
Soluble in water.
Conduct electricity when dissolved or melted.
Covalent Compounds (e.g., H₂O)
Low melting and boiling points.
Often insoluble in water.
Do not conduct electricity.
INTEXT – QUESTION- 1
1) How do atoms attain noble gas configuration?
Ans: Atoms become stable by gaining, losing, or sharing electrons to achieve a full outer electron shell, like noble gases.
For example, sodium (Na) loses an electron to resemble neon (Ne), and chlorine (Cl) gains one to match argon (Ar).
In covalent bonding, atoms share electron pairs. Two oxygen atoms, for instance, share two electrons each to complete their outer shells.
This need for a full outer shell (usually 8 electrons, called an octet) is the main driver of chemical bonding.
2) Define:
(a) a chemical bond
(b) an electrovalent bond
(c) a covalent bond
Ans: (a) A chemical bond is the powerful force that acts like glue, holding atoms together to form molecules or compounds. Atoms bond to become more stable, achieving this by either sharing or transferring their outermost electrons to reach a lower, more stable energy state.
(b) Electrovalent Bond
A bond formed by the complete transfer of electrons from a metal to a non-metal, resulting in oppositely charged ions attracted electrostatically.
(c) Covalent Bond
A bond where non-metal atoms share electron pairs to achieve stable configurations. It can be single, double, or triple.
3) What are the conditions for the formation of an electrovalent bond?
Ans: An electrovalent (ionic) bond forms when one atom transfers an electron to another, creating oppositely charged ions held together by strong electrostatic attraction.
The key conditions for its formation are:
Large Electronegativity Difference: A major difference (typically >1.7) between the atoms.
Low Ionization Energy: The metal atom must easily lose an electron to form a cation.
High Electron Affinity: The non-metal atom must readily gain an electron to form an anion.
Favorable Lattice Energy: Significant energy is released when the ions form a stable, solid crystal lattice.
4) An atom X has three electrons more than the noble gas configuration. What type of ion will it form? Write the formula of its
(i) sulphate
(ii) nitrate
(iii) phosphate
(iv) carbonate
(v) hydroxide
Ans: It will form a cation : M3+
M2(SO4)3
M(NO3)3
M3(PO4)3
M2(CO3)3
M(OH)3
5) Mention the basic tendency of an atom which makes it to combine with other atoms.
Ans: An atom’s fundamental drive to bond with others comes from its need to achieve a stable electron arrangement, similar to the noble gases. This is often called the octet rule, where atoms aim for eight electrons in their outer shell.
They achieve this stability in three primary ways:
Losing Electrons: Metal atoms (like sodium) lose their outer electrons to empty that shell, leaving a full shell underneath.
Gaining Electrons: Non-metal atoms (like chlorine) gain electrons to fill their outer shell and complete the octet.
Sharing Electrons: Non-metal atoms can share pairs of electrons with each other, allowing both atoms to complete their outer shells.
6) What type of compounds are usually formed between metals and non-metals and why?
Ans: Compounds made from a metal and a non-metal are usually ionic. This happens because of how differently these elements handle electrons.
Metals, found on the left side of the periodic table, have only a few electrons in their outer shell. They easily lose these electrons to achieve stability, forming a positive ion (a cation).
Non-metals, on the right side, have nearly full outer shells. They strongly gain electrons to become stable, forming a negative ion (an anion).
This complete hand-off of electrons from the metal to the non-metal creates oppositely charged ions.
7) In the formation of the compound XY2, an atom X gives one electron to each Y atom. What is the nature of bond in XY2? Draw the electron dot structure of this compound.
Ans: This is because the bond is formed by the complete transfer of electrons from metal atom X to non-metal atoms Y.
Electron Dot Structure of XY₂:
Atom X, after losing two electrons, becomes a cation (X²⁺).
Each atom Y, after gaining one electron, becomes an anion (Y⁻).The structure is represented as:
..
[ : Y : ]⁻ ²⁺ [ : Y : ]⁻
.. ..
8) An atom X has 2, 8, 7 electrons in its shell. It combines with Y having 1 electron in its outermost shell.
(a) What type of bond will be formed between X and Y?
(b) Write the formula of the compound formed.
Ans: (a) Ionic bond
(b) Formula: YX
X gains an electron, Y loses one. This creates Y⁺ and X⁻ ions that attract each other, like in NaCl
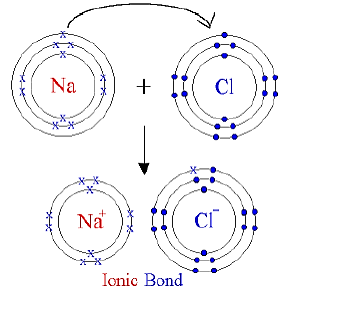
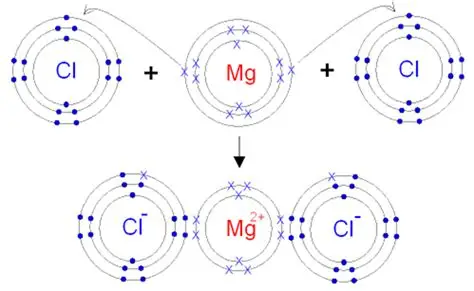
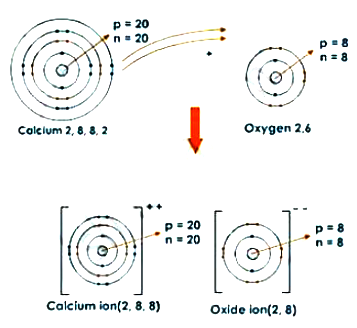
9) Draw orbit structure and electron dot diagram of NaCI, MgCl2 and CaO.
Ans: Orbit structure and electron dot diagram of NaCl:
Orbit structure and electron dot diagram of MgCl2:
Orbit structure and electron dot diagram of CaO:
10) Compare:
(a) sodium atom and sodium ion
(b) chlorine atom and chloride ion, with respect to
(i) atomic structure
(ii) electrical state
(iii) chemical action
(iv) toxicity
Ans: (a) Sodium Atom vs. Sodium Ion
| Ascept | Sodium Atom | Sodium Ion |
| (i) Atomic Structure | Has 17 protons and 17 electrons. The electron configuration is 2,8,7. It has 7 electrons in its outermost shell. | Has 17 protons but 18 electrons. The electron configuration is 2,8,8. It has a stable octet |
| (ii) Electrical State | Electrically neutral (protons = electrons). | Negatively charged (anion) because it has one more electron than protons. |
| (iii) Chemical Action | Extremely reactive and a powerful oxidizing agent. It is a poisonous greenish-yellow gas. | Chemically stable and unreactive. It is the form that combines with sodium to form stable, non-reactive table salt (NaCl). |
| (iv) Toxicity | Highly toxic and corrosive as a gas. It was used as a chemical weapon. | Mostly non-toxic. It is a crucial ion for nerve function and digestion in the body. |
(b) Chlorine Atom vs. Chloride Ion
| Ascept | Chlorine Atom | Chloride Ion |
| (i) Atomic Structure | Has 17 protons and 17 electrons. The electron configuration is 2,8,7. It has 7 electrons in its outermost shell. | Has 17 protons but 18 electrons. The electron configuration is 2,8,8. It has a stable octet. |
| (ii) Electrical State | Electrically neutral (protons = electrons). | Negatively charged (anion) because it has one more electron than protons. |
| (iii) Chemical Action | Extremely reactive and a powerful oxidizing agent. It is a poisonous greenish-yellow gas. | Chemically stable and unreactive. It is the form that combines with sodium to form stable, non-reactive table salt (NaCl). |
| (iv) Toxicity | Highly toxic and corrosive as a gas. It was used as a chemical weapon. | Mostly non-toxic. It is a crucial ion for nerve function and digestion in the body. |
11) The electronic configuration of fluoride ion is the same as that of a neon atom. What is the difference between the two?
Ans: Although the fluoride ion (F⁻) and the neon (Ne) atom have the same electron configuration (2,8), they are fundamentally different.
The key difference is in their nucleus (atomic structure):
| Features | Fluoride Ion (F⁻) | Neon Atom (Ne) |
| Number of Protons | 9 protons | 10 protons |
| Nuclear Charge | Weaker positive charge (+9) | Stronger positive charge (+10) |
| Nature | A negatively charged ion | A neutral atom |
In short, F⁻ has fewer protons trying to pull in the same number of electrons as Ne. This makes F⁻ an unstable ion that readily reacts, while Ne is a stable and inert noble gas.
INTEXT – QUESTION- 2
1) What are the conditions necessary for the formation of covalent molecules?
Ans: Non-Metal Atoms: The bonding must occur between two or more non-metal atoms.
Electron Sharing: Each atom must have an incomplete valence shell and a high tendency to gain electrons (high ionization energy/electron affinity), making electron transfer difficult. Instead, they share electrons to achieve stability.
Stable Configuration: The shared electrons must help each atom attain a stable noble gas electron configuration (octet or duet).
2) Elements A, B and C have atomic number 17, 19 and 10 respectively.
(a) State which one is: (i) a non-metal (ii) a metal, (iii) chemically inert?
(b) write down the formula of the compound formed by two of the above elements.
(a) A is a non-metal; B is a metal while C is a chemically inert element.
(b) BA
Ans: (a) Identification of Elements:
(i) Non-metal: This is element A (Chlorine). It has 7 valence electrons and tends to gain an electron.
(ii) Metal: This is element B (Potassium). It has 1 valence electron and tends to lose it.
(iii) Inert element: This is element C (Neon). It has a complete octet (8 valence electrons) and is chemically unreactive.
(b) Compound Formation:
The compound formed is BA (chemical formula: KCl).
Explanation:
Element B (Potassium, a metal) readily loses its single valence electron. Element A (Chlorine, a non-metal) gains that electron. This electron transfer results in the formation of ions B⁺ (K⁺) and A⁻ (Cl⁻), which are held together by a strong ionic bond. Element C (Neon) is a noble gas with a stable electron configuration, so it does not gain, lose, or share electrons and does not form a compound.
3) Five atoms are labelled from A to E.
| Atoms | Mass No. | Atomic No. |
| A | 40 | 20 |
| B | 19 | 9 |
| C | 7 | 3 |
| D | 16 | 8 |
| E | 14 | 7 |
(a) Which one of these atoms:
(i) contains 7 protons,
(ii) has an electronic configuration 2, 7?
(b) Write down the formula of the compound formed between C and D.
(c) predict which are:
(i) metals
(ii) non-metals?
Ans:
(a) (i) E (ii) B
(b) C2D
(c) A and C are metals while B, D and E are non -metals.
4) What is the difference between:
(a) ionic compounds and polar covalent compounds,
(b) ionic compounds and covalent compounds,
(c) a polar covalent compound and a non-polar covalent compound?
Ans: (a) Ionic Compounds vs. Polar Covalent Compounds
The key difference lies in how electrons are shared between atoms. Ionic compounds are formed by the complete transfer of electrons from a metal to a non-metal, creating positive and negative ions that attract each other. There is no sharing. In contrast, polar covalent compounds are formed between different non-metals where electrons are shared, but unequally. This unequal sharing creates partial positive and negative charges (dipoles) within the molecule, but no full ions are formed.
(b) Ionic and covalent compounds are defined by their bonding mechanisms. Ionic bonds form when a metal atom donates electrons to a non-metal, creating positive and negative ions. These ions are held together by powerful electrostatic attraction, leading to high melting points, and the ability to conduct electricity when dissolved or molten.
Conversely, covalent bonds occur between non-metal atoms that share electron pairs to achieve stability. This results in discrete molecules. They cannot conduct electricity as they lack free ions or electrons.
(c) Polar Covalent vs. Non-Polar Covalent Compounds
Both involve electron sharing, but the key difference is the electronegativity difference between the bonded atoms. In a polar covalent bond, atoms with different electronegativities share electrons unequally, creating a dipole moment (e.g., H₂O). In a non-polar covalent bond, atoms with identical or very similar electronegativities share electrons equally, resulting in no separation of charge (e.g., O₂ or CH₄).
5) The element X has the electronic configuration 2, 8, 18, 8, 1. Without identifying x,
(a) predict the sign and charge on a simple ion of X.
(B) write if X is an oxidizing agent or reducing agent and why.
Ans: (a) Sign and Charge:
The ion of element X has a positive (+) sign and a charge of +1, written as X⁺.
Why?
Its electron configuration ends with …8, 1. This single electron in the outermost shell is easily lost to achieve a stable, full outer electron shell (2,8,18,8), which is a stable configuration.
(b) Oxidizing or Reducing Agent?
X is a reducing agent.
Why?
By losing its outermost electron easily (to form X⁺), it causes another substance to gain that electron and be reduced. Since it donates an electron, it itself is oxidized and acts as a reducing agent.
6) What do you understand about polar covalent compounds? Explain it by taking hydrogen chloride as an example.
Ans: What are Polar Covalent Compounds?
Polar covalent compounds are molecules where atoms share electrons, but not equally. This happens because one atom has a stronger pull on the shared electrons than the other. This unequal sharing creates a small separation of charge, making one end of the molecule slightly positive and the other end slightly negative. We call this a “dipole”.
Explanation using Hydrogen Chloride (HCl) as an example:
The Bond: In HCl, a hydrogen (H) atom and a chlorine (Cl) atom share a pair of electrons to form a covalent bond.
The Result: The shared electron pair is pulled closer to the chlorine atom and away from the hydrogen atom.
Creating Poles:
Because the electrons spend more time near chlorine, that end of the molecule becomes slightly negative (δ-).
The hydrogen end, having lost some electron presence, becomes slightly positive (δ+).
So, an HCl molecule is polar because it has two opposite poles, just like a magnet has a north and south pole. This polarity influences its properties, like its high solubility in water.
7) Methane molecule is non-polar molecule. Explain.
Ans: A methane (CH₄) molecule is non-polar for two main reasons:
This means the electrons in each C-H bond are shared equally, so no significant partial charges form.
Symmetrical Shape: Methane has a perfect tetrahedral shape. Imagine the carbon atom at the center with four hydrogen atoms at the four corners.
While each individual C-H bond is very slightly polar, the symmetrical shape means these tiny pulls cancel each other out in all directions. It’s like four people pulling on the center of a rope with identical strength; the net pull is zero.
Because there is no overall shift of electron charge to one side, the entire molecule has no separate positive or negative ends, making it non-polar.
8) Give the characteristic properties of:
(a) electrovalent compounds,
(b) covalent compounds.
Ans: Properties of Ionic Compounds
State: They are hard, brittle solids with a crystalline structure.
Melting/Boiling Points: They have very high melting and boiling points.
Solubility: They usually dissolve well in polar solvents like water but not in non-polar solvents like petrol.
Conductivity: They cannot conduct electricity as solids. They only conduct when melted or dissolved in water.
Reactions: Their reactions in solutions are very fast.
Properties of Covalent Compounds
State: They can be gases, liquids, or soft solids.
Melting/Boiling Points: They generally have low melting and boiling points.
Solubility: They are usually soluble in non-polar solvents like benzene but are insoluble in water.
Conductivity: They do not conduct electricity in any state (solid, liquid, or dissolved).
Reactions: Their reactions are typically slow.
9) (a) What do you understand redox reactions? Explain oxidation and reduction in terms of loss or gain of electrons.
(b) Divide the following redox reactions into oxidation and reduction half reactions.
(i) Zn + Pb2 → Zn2 + Pb
(ii) Zn + cu2 → Zn2 + Cu
(iii) CI2 + 2Br → Br2 + 2CI-
(iv) Sn2 + 2Hg2 → Sn4 + Hg22+
(v) 2Cu+ → Cu + Cu2+
(c) Potassium (at No .19) and chlorine (at No. 17) react to form a compound.
Explain the basis of the electronic concept.
(i) oxidation
(ii) reduction
(iii) oxidizing agent
(iv) reducing agent
Ans:
a)What Are Redox Reactions?
A redox reaction is a type of chemical reaction where the oxidation states of atoms are changed. It involves the transfer of electrons between two species. The term “redox” is a portmanteau of reduction and oxidation.
b) Oxidation is the loss of electrons by a substance. When a substance is oxidized, its oxidation number increases.Reduction is the gain of electrons by a substance. When a substance is reduced, its oxidation number decreases.A simple way to remember this is the acronym OIL RIG: Oxidation Is Loss, Reduction Is Gain of electrons.Oxidation and reduction always occur together because the electrons lost by one substance must be gained by another. One substance can’t just lose electrons into thin air; they must be accepted by something else.
Oxidation and Reduction Half-Reactions
Here are the given redox reactions divided into their respective half-reactions:
(i) Zn+Pb 2+→Zn 2+ +Pb
Oxidation: Zn→Zn 2++2e −
(Zinc loses electrons)
Reduction: Pb2++2e − →Pb (Lead gains electrons)
(ii) Zn+Cu 2+ →Zn 2++Cu
Oxidation: Zn→Zn2++2e −
(Zinc loses electrons) Reduction: Cu2++2e − →Cu (Copper gains electrons)
(iii) Cl2+2Br −→Br 2+2Cl −
Oxidation: 2Br −→Br2+2e −
(Bromide loses electrons)Reduction: Cl2+2e −→2Cl −(Chlorine gains electrons)
(iv) Sn 2++2Hg 2+→Sn 4++Hg 22+
Oxidation: Sn2+→Sn 4++2e −
(Tin loses electrons)Reduction: 2Hg2++2e −→Hg 22+(Mercury gains electrons)
(v) 2Cu +→Cu+Cu 2+
Oxidation: Cu +→Cu 2++e
(One copper ion loses an electron)Reduction: Cu ++e −→Cu (Another copper ion gains an electron)
c) Electronic Concept of Potassium and Chlorine Reaction
Potassium (atomic number 19) and chlorine (atomic number 17) react to form the ionic compound potassium chloride (KCl). Their electronic configurations are:
Potassium (K): 2, 8, 8, 1 (one valence electron)
Chlorine (Cl): 2, 8, 7 (seven valence electrons)
The reaction occurs as a transfer of an electron from the potassium atom to the chlorine atom, resulting in both atoms achieving a stable, full outer electron shell.Potassium loses its single valence electron to become a potassium ion (K +), which now has a stable configuration of 2, 8, 8.Chlorine gains that electron to become a chloride ion (Cl −), achieving a stable configuration of 2, 8, 8.
(i) Oxidation: The potassium atom is oxidized because it loses an electron. The reaction is K→K++e−.
(ii) Reduction: The chlorine atom is reduced because it gains an electron. The reaction is Cl+e −→Cl −.
(iii) Oxidizing Agent: The chlorine atom is the oxidizing agent. It causes the oxidation of potassium by accepting the electron, thus getting itself reduced.
(iv) Reducing Agent: The potassium atom is the reducing agent. It causes the reduction of chlorine by donating its electrons, thus getting itself oxidized.
10) What do you understand about a dipole molecule? Give one example.
Ans: A dipole molecule, also known as a polar molecule, is a neutral molecule that has a separation of positive and negative electrical charge. This occurs when there is an uneven distribution of electrons between the atoms in a molecule, often because one atom is more electronegative (has a stronger pull on electrons) than the other. This unequal sharing creates a positive end and a negative end, resulting in a net dipole moment.
An excellent example of a dipole molecule is water (H2O).
In a water molecule, the oxygen atom is more electronegative than the two hydrogen atoms. This causes the shared electrons to be pulled closer to the oxygen atom, giving it a partial negative charge (δ−), while the hydrogen atoms are left with a partial positive charge (δ+). Because the water molecule has a bent shape, these partial charges don’t cancel each other out, creating a net dipole moment
INTEXT – QUESTION- 3
1) Explain the following:
(a) Electrovalent compounds conduct electricity.
(b) Electrovalent compounds have a high melting point and boiling point while covalent compounds have low melting and boiling points.
(c) Electrovalent compounds dissolve in water whereas covalent compounds do not.
(d) Electrovalent compounds are usually hard crystals yet brittle.
(e) polar covalent compounds electricity.
Ans: (a) Electrovalent compounds conduct electricity.
Electrovalent compounds, also known as ionic compounds, are formed by the complete transfer of electrons between atoms, creating positive and negative ions. In their solid state, these ions are held in a fixed, rigid crystal lattice and cannot move, so they don’t conduct electricity. However, when they are melted (molten) or dissolved in a solvent like water, the ions are free to move. This mobility of charged particles (ions) allows the substance to conduct an electric current.
(b) Electrovalent vs. Covalent Compounds: Melting and Boiling Points.
- Electrovalent compounds have a high melting and boiling point due to the strong electrostatic forces of attraction between oppositely charged ions. A significant amount of energy is needed to overcome these strong forces and break the crystal lattice, allowing the substance to melt or boil.
- Covalent compounds have a low melting and boiling point because they are made of discrete molecules. The forces holding these molecules together (intermolecular forces) are much weaker than the electrostatic forces in ionic compounds. Therefore, less energy is required to overcome these weak forces, resulting in lower melting and boiling points.
(c) Electrovalent vs. Covalent Compounds: Solubility in Water.
- Electrovalent compounds dissolve in water because water is a polar solvent. The positive end of a water molecule attracts the negative ion of the ionic compound, and the negative end of a water molecule attracts the positive ion. This interaction, known as ion-dipole interaction, is strong enough to pull the ions out of the crystal lattice, causing the compound to dissolve.
- Covalent compounds are generally non-polar and do not dissolve in water because there are no strong attractions between their molecules and the polar water molecules. The energy required to break the covalent bonds and overcome the intermolecular forces is too high for water to supply, so the compound remains undissolved.
(d) Electrovalent compounds are hard yet brittle.
Electrovalent compounds are typically hard because of the strong electrostatic forces of attraction holding the ions in a rigid, repeating crystalline structure. This strong attraction prevents the ions from being easily displaced. However, they are also brittle. If a force is applied to the crystal, it can cause the layers of ions to shift. This shift brings ions of the same charge into close proximity, resulting in a strong repulsion that can cause the crystal to cleave or shatter.
(e) Polar covalent compounds conduct electricity.
Polar covalent compounds, such as hydrogen chloride (HCl), do not conduct electricity in their pure state because they are composed of neutral molecules. However, when dissolved in a polar solvent like water, some polar covalent molecules can ionize. For example, HCl in water forms hydronium ions (H3O+) and chloride ions (Cl−). The presence of these free-moving ions allows the solution to conduct electricity.
2)A solid is crystalline, has a high melting point and is water soluble. Describe the nature of the solid.
Ans: The solid described is an ionic compound. This is because its properties—being crystalline, having a high melting point, and dissolving in water—are characteristic of ionic substances.
- Crystalline: The regular, ordered arrangement of positive and negative ions in an ionic compound forms a crystal lattice.
- High Melting Point: A large amount of energy is needed to break the strong electrostatic forces holding the ions together, which results in a high melting point.
- Water Soluble: Water is a polar solvent, so its molecules are attracted to and can separate the oppositely charged ions from the lattice, allowing the compound to dissolve.
3) Match the atomic number 4, 8, 14, 15 and 19 with each of the following:
(a) A solid non-metal of valency 3.
(b) A gas of valency 2.
(c) A metal of valency 1.
(d) A non-metal of valency 4
Ans: (a) Phosphorus (P): A solid non-metal with a valency of 3. With an atomic number of 15 and electron configuration 2, 8, 5, it needs to gain 3 electrons to complete its octet, making its valency 3. At room temperature, it’s solid.
(b) Oxygen (O): A gas with a valency of 2. Its atomic number is 8, and its electron configuration is 2, 6. It needs to gain 2 electrons to fill its outer shell, so its valency is 2.
(c) Potassium (K): A metal with a valency of 1. With an atomic number of 19 and electron configuration 2, 8, 8, 1, it readily loses its single valence electron to achieve a stable octet, giving it a valency of 1. It is a solid, alkali metal at room temperature.
(d)Silicon (Si): A non-metal with a valency of 4. With an atomic number of 14 and an electron configuration of 2, 8, 4, silicon tends to share its four valence electrons to achieve a stable octet, making its valency 4. It’s classified as a metalloid and is a solid at room temperature.
4) Elements X, Y, and Z have atomic numbers 6, 9 and 12 respectively. Which one:
(a) forms an anion,
(b) forms a cation,
(c) State type of bond between Y and Z and give its molecular formula.
Ans: (a) Y
(b) Z
(c) X
5) Taking MgCI2 as an electrovalent compound, CCI4 as a covalent compound, give four differences between electrovalent and covalent compounds.
Ans: Differences Between Electrovalent and Covalent Compounds
Electrovalent compounds, such as Magnesium Chloride (MgCl₂), and covalent compounds, such as Carbon Tetrachloride (CCl₄), differ fundamentally in their structure and properties.
Firstly, their formation and bonding nature are opposite. Electrovalent compounds are formed by the complete transfer of electrons from a metal to a non-metal, creating positive and negative ions held by strong electrostatic forces. For example, magnesium atoms donate electrons to chlorine atoms to form MgCl₂. In contrast, covalent compounds are formed by the mutual sharing of electron pairs between two non-metal atoms. In CCl₄, carbon and chlorine atoms share electrons to achieve stable electron configurations.
Secondly, their physical states differ significantly at room temperature. Due to their strong ionic bonds forming a giant lattice structure, electrovalent compounds like MgCl₂ are almost always solid, hard, and brittle. Covalent compounds like CCl₄, with weaker intermolecular forces between their molecules, can often be found as liquids or gases; CCl₄ is a liquid at room temperature.
Thirdly, their solubility follows a distinct pattern. Electrovalent compounds are generally soluble in polar solvents like water because the water molecules can stabilize the charged ions. Covalent compounds are typically insoluble in water but dissolve well in non-polar organic solvents like benzene or kerosene. For instance, CCl₄ mixes easily with these solvents but not with water.
Finally, their ability to conduct electricity is a major differentiator. Electrovalent compounds in a molten state or when dissolved in water can conduct electricity, as their ions become free to move and carry charge. Solid MgCl₂ does not conduct, but its solution does. Covalent compounds like liquid CCl₄ do not conduct electricity at all in any state, as they possess no free ions or electrons.
6)Potassium chloride is an electrovalent compound, while hydrogen chloride is a covalent compound. But, both conduct electricity in their aqueous solutions. Explain.
Ans: Potassium chloride (KCl) and hydrogen chloride (HCl) both conduct electricity when dissolved in water because both solutions contain free-moving ions, which are essential for carrying an electric current.
For potassium chloride (KCl), an ionic compound, the solid is already composed of K+ and Cl− ions held in a crystal lattice. When dissolved in water, the polar water molecules break apart this lattice, freeing the existing ions to move and conduct electricity. This process is known as dissociation.
For hydrogen chloride (HCl), a polar covalent compound, it exists as neutral molecules in its pure state. However, when dissolved in water, the water molecules are strong enough to pull the hydrogen atom’s proton away from the chlorine atom. This creates separate, mobile ions—the hydronium ion (H3O+) and the chloride ion (Cl−)—which can then conduct electricity. This process is called ionization.
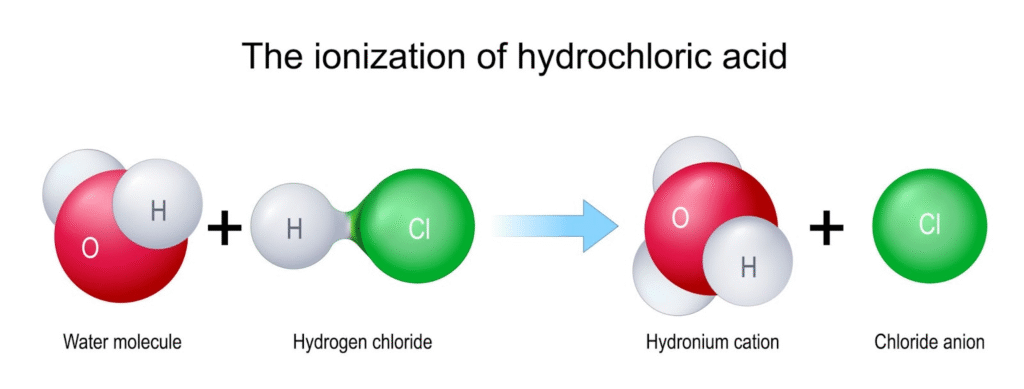
In short, while KCl starts with ions that are freed in water, HCl creates ions when it dissolves. Both result in an electrolytic solution that can conduct electricity.
7) (a) Name two compounds that are covalent when taken pure but produce ions when dissolved in water.
(b) For each compound mentioned above give the formulae of ions formed in aqueous solution.
Ans: (a) The two compounds that are covalent when pure but produce ions when dissolved in water are hydrogen chloride (HCl) and sulfuric acid (H2SO4). They are considered strong acids.
(b) The formulae of the ions formed by these compounds in an aqueous solution are as follows:
- Hydrogen Chloride (HCl):
- H3O+ (hydronium ion)
- Cl− (chloride ion)
- Sulfuric Acid (H2SO4):
- H3O+ (hydronium ion)
- HSO4− (hydrogen sulfate ion)
- SO42− (sulfate ion)
8) An element M burns in oxygen to form an ionic bond MO. Write the formula of the compounds formed if this element is made to combine with chlorine and sulphur separately.
Ans:
When element M burns in oxygen to form MO, it indicates that M has a valency of +2.
With chlorine (valency -1), the compound formed is MCl₂.
With sulphur (valency -2), the compound formed is MS.
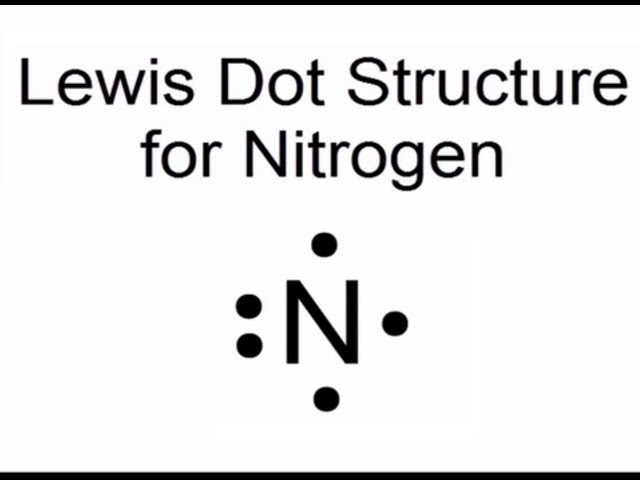
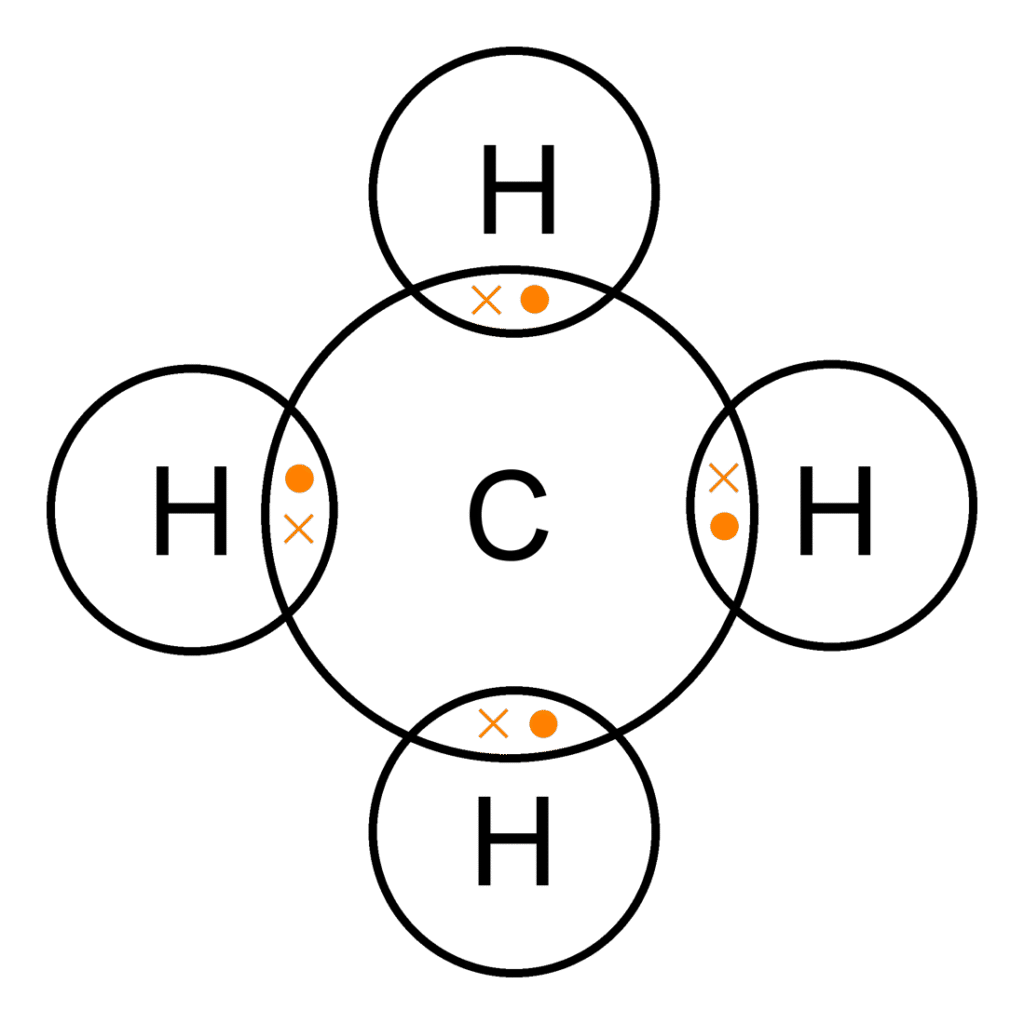
Question 9: Give electron dot diagram of the following:
(a) Magnesium chloride
(b) nitrogen
(c) methane
Ans:
a)
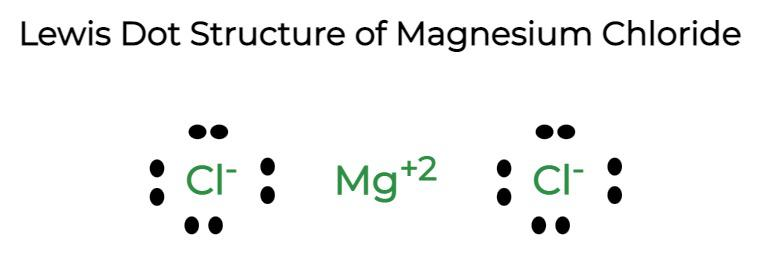
b)
c)
10) State the type of bonding in the following molecules.
(a) Water
(b) Calcium oxide
(c) hydroxyl ion,
(d) methane,
(e) ammonium ion
(f) ammonium chloride
Ans: (a) Water (H2O)
Water has covalent bonding. An oxygen atom shares its electrons with two hydrogen atoms to form two single covalent bonds.
(b) Calcium Oxide (CaO)
Calcium oxide has ionic bonding. Calcium (Ca) is a metal that transfers two electrons to oxygen (O), a non-metal. This forms a Ca 2+cation and an O2−anion, which are attracted to each other by electrostatic forces.
(c) Hydroxyl ion (OH−)
The hydroxyl ion has covalent bonding. The oxygen and hydrogen atoms share electrons to form a single bond. The overall charge of the ion is due to an extra electron.
(d) Methane (CH 4)
Methane has covalent bonding. A carbon atom shares electrons with four hydrogen atoms to form four single covalent bonds.
f) Ammonium chloride (NH₄Cl) is held together by ionic bonding between the ammonium ions (NH₄⁺) and chloride ions (Cl⁻).
Within each ammonium ion, the atoms are bonded by covalent bonds. One of these four N-H bonds is a dative covalent (coordinate) bond, formed when the nitrogen atom in ammonia (NH₃) donates its lone pair to a hydrogen ion (H⁺). After formation, all four bonds are identical.
In short: The overall structure is ionic, while the bonds inside the NH₄⁺ ion are covalent.
11) Define a coordinate bond and give the conditions for its formation. Explain with an example.
Ans: A coordinate bond, or dative bond, is a type of covalent bond where one atom donates both of the electrons that are shared between two atoms. Unlike a typical covalent bond, where each atom contributes one electron, a coordinate bond is formed when a donor atom with a lone pair of electrons shares that pair with an acceptor atom that has an empty orbital. This process allows for the formation of a stable bond.
A classic example of this is the formation of the ammonium ion (NH4+) from ammonia (NH3) and a hydrogen ion (H+). The nitrogen atom in ammonia has a lone pair of electrons, making it the donor. The hydrogen ion, which is simply a proton, has an empty 1s orbital, allowing it to act as the acceptor. The lone pair from the nitrogen is donated to the hydrogen ion’s empty orbital, forming a new bond. Once the NH4+ion is formed, all four N-H bonds are identical, demonstrating that a coordinate bond is fundamentally a type of covalent bond. This highlights how a coordinate bond, though formed uniquely, results in a stable chemical structure indistinguishable from one formed by standard covalent bonding.
12) (a) What do you understand by lone pair and shared pair?
(b) (i) How many atoms of each kind are present in the following molecules: calcium oxide, chlorine, water, carbon tetrachloride?
(ii) How many electrons are required for their octet structure?
Ans: (a) A lone pair is a pair of valence electrons that are not involved in a chemical bond. They belong exclusively to a single atom. A shared pair, also known as a bonding pair, is a pair of valence electrons that are shared between two atoms to form a covalent bond.
b)(i)Calcium oxide (CaO) is a simple ionic compound. The ratio of calcium to oxygen atoms is 1:1. Chlorine gas is a diatomic molecule, represented as Cl2, which means it consists of two chlorine atoms bonded together. Water (H2O) is a molecule with a single oxygen atom covalently bonded to two hydrogen atoms. Finally, carbon tetrachloride (CCl4) is a molecule where one central carbon atom is bonded to four chlorine atoms.
(ii) Electrons Required for Octet:
The number of electrons required for an octet is determined by the number of atoms that need a complete valence shell. Hydrogen is an exception, as it only requires 2 electrons (a duet) to achieve a stable configuration.
Calcium oxide: The calcium ion (Ca²⁺) has an octet, and the oxygen ion (O²⁻) also has an octet. Together, they require a total of 8 electrons to form these stable ions from their neutral atoms.
Chlorine (Cl₂): Each chlorine atom requires 1 electron to complete its octet. As they share one pair of electrons, the molecule requires a total of 2 electrons to achieve the octet for both atoms.
Water (H₂O): The oxygen atom requires 8 electrons for an octet, and each hydrogen atom requires 2. The oxygen shares electrons with both hydrogens. In total, 8 electrons are needed for the oxygen’s octet and 4 electrons (2 for each H) for the hydrogens’ duets.
Carbon tetrachloride (CCl₄): The carbon atom requires 8 electrons for its octet, and each of the four chlorine atoms requires 8. Through shared electron pairs, this structure is achieved. The total electrons required for all atoms to achieve an octet (or duet) is 40 electrons (8 for C + 8×4 for Cl).
13) Complete the following:
(a) When the nuclei of two different reacting atoms are of ……………… mass, then a bond so formed is called ……………… covalent band (Equal, unequal, polar, non-polar).
(b) In case of non-polar covalent bond, the covalent bond is formed in the ……………. Of atoms and shared electrons are …………. Distributed (corner, middle, equally, unequally).
(c) The ions in ……………… compounds are held very strongly due strong ……. Forces (electrovalent, covalent, electromagnetic, electrostatic).
Ans: (a) Unequal, polar
(b) Middle, equally
(c) Electrovalent, electrostatic
14) (a) Draw an electron dot diagram to show the structure of each of the following:
(i) Hydronium ion,
(ii) Ammonium ion,
(iii) Hydroxyl ion.
State the type of bonding present in them.
Ans: (i) The hydronium ion (H₃O⁺) features a central oxygen atom bonded to three hydrogen atoms. Oxygen donates its lone pair to form a coordinate covalent bond with an additional proton (H⁺), which gives the entire ion its positive charge.
(ii) Similarly, in the ammonium ion (NH₄⁺), a central nitrogen atom is bonded to four hydrogen atoms. Nitrogen uses its lone pair to create a coordinate covalent bond with one hydrogen ion (H⁺), while the other three are standard covalent bonds. This structure results in the ion’s overall positive charge.
(iii) Hydroxyl ion (OH⁻): Its structure consists of an oxygen atom bonded to a hydrogen atom. Oxygen has three lone pairs of electrons, and the ion is enclosed in a square bracket with a negative charge. The bonding between the oxygen and hydrogen is a simple covalent bond.
(b) Give two examples in each case:
(i) Co-ordinate bonds compounds,
(ii) solid covalent compounds,
(iii) Gaseous polar compounds,
(iv) Gaseous non polar compounds,
(v) Liquid non polar compounds.
Ans:
(i) In coordinate covalent bonding, one atom fully donates a shared pair of electrons to form a bond. This is clearly illustrated in two common examples. The ammonium ion (NH₄⁺) forms when a nitrogen atom provides both electrons to bond with a hydrogen ion (H⁺). Similarly, in the ozone molecule (O₃), one oxygen atom donates an electron pair to another, which is a key reason behind its characteristic bent molecular shape.
(ii) Solid covalent compounds form giant atomic structures held together by a continuous network of strong covalent bonds, which requires considerable energy to break. This gives them exceptionally high melting points. Notable examples include diamond, where each carbon atom is tetrahedrally bonded to four others, and silicon dioxide (SiO₂), or quartz, which has a similar robust crystalline framework.
(iii) Gaseous Polar Compounds
These are gases whose molecules have an uneven distribution of charge due to differences in electronegativity. Two clear examples are hydrogen chloride (HCl), where the chlorine atom pulls electron density toward itself, and ammonia (NH₃), which has a pyramidal shape leading to a significant dipole moment.
(iv) Gaseous Non-Polar Compounds
These gas molecules have symmetrical shapes and atoms with similar electronegativities, resulting in no net dipole. Common examples include oxygen gas (O₂), with an identical atom sharing electrons equally, and methane (CH₄), whose tetrahedral symmetry cancels out any individual bond polarities.
(v) Liquid Non-Polar Compounds
These are liquids composed of molecules with no permanent dipole moment. Widespread examples include benzene (C₆H₆), a symmetrical ring-shaped hydrocarbon, and hexane (C₆H₁₄), a common non-polar solvent where the carbon and hydrogen atoms share electrons almost equally.
15) Element M forms a chloride with the formula MCI2 which is a solid with high melting point. M would most likely be in the group in which ………….. is placed. [ (a) Na (b) Mg (c) Al (d) Si ]
Ans: Mg
16) Complete the following:
| Sodium | Phosphorus | Carbon | |
| Formula of chloride | |||
| Nature of bonding | |||
| Physical state of chloride |
Ans:
| Sodium | Phosphorus | Carbon | |
| Formula of chloride | NaCl | PCl5 | CCl4 |
| Nature of bonding | Ionic | Covalent | Covalent |
| Physical state of chloride | Solid | Solid | Liquid |
Question (2004): (a) Element X is a metal with a valency 2. Element Y is a non-metal with a valency 3.
(i) Write equations to show how X and Y form ions.
(ii) If Y is a diatomic gas, write the equation for the direct combination of X and Y to form a compound.
(iii) If the compound formed between X and Y is melted and an electric current is passed through the molten compound, the element X will be obtained at the ……… and Y at the ……….. of the electrolytic cell.
Ans: (i) Element X, a metal with valency 2, loses two electrons to form a positive ion: X → X²⁺ + 2e⁻. Element Y, a non-metal with valency 3, gains three electrons to form a negative ion: Y + 3e⁻ → Y³⁻.
(ii) If Y is a diatomic gas (Y₂), the direct combination with X is represented by the equation: 3X + Y₂ → X₃Y₂.
(iii) When the molten compound is electrolyzed, element X (the metal) will be obtained at the cathode, and Y (the non-metal) will be obtained at the anode of the electrolytic cell. This occurs because the positive metal ions (X²⁺) are attracted to the negative cathode, where they gain electrons, while the negative non-metal ions (Y³⁻) are attracted to the positive anode, where they lose electrons.
Question (2005): (a) Compound X consists of molecules. Choose the letter corresponding to the correct answer from the options A,B,C and D given below:
(i) The type of bonding in X will be:
A. ionic
B. electrovalent
C. covalent
D. molecular
(ii) X is likely to have a:
A. low melting point and high boiling point,
B. high melting point and low boiling point
C. Low melting point and low boiling point,
D. high melting point and high boiling point.
(iii) In the liquid state, X will:
A. become ionic
B. be an electrolyte
C. conduct electricity
D. not conduct electricity
Ans: (a) The compound X consists of molecules.
(i) The type of bonding in X will be:
C. covalent
The statement specifies that the substance is made of molecules. This is the key characteristic of covalent compounds, where atoms share electrons to form distinct molecules.
(ii) X is likely to have a:
C. Low melting point and low boiling point
Substances with covalent molecular bonding are held together by weak intermolecular forces, not strong ionic or covalent bonds throughout a giant lattice. These weak forces require very little energy to overcome, resulting in low melting and boiling points.
(iii) Substance X does not conduct electricity in its liquid state because it is a covalent molecular substance. This type of substance consists of neutral molecules that have no overall charge. Crucially, it lacks the free-moving charged particles—such as ions or delocalized electrons—that are essential for conducting an electrical current. Since the electrons are held tightly within the bonds of the molecules and are not free to move, an electric current cannot flow through the liquid.
(b) Electrons are getting added to an element Y:
(i) is Y getting oxidized or reduced?
(ii) what charge will Y migrate to during the process of electrolysis?
(i) Is Y getting oxidized or reduced?
Y is getting reduced because it is gaining electrons.
(ii) What charge will Y migrate to during electrolysis?
Y will become negatively charged (anion) and migrate to the positive electrode (anode).
(c) (i) Acids dissolve in water and produce positively charged ions. Draw the structure of these positive ions.
(ii) Explain why carbon tetrachloride does not dissolve in water.
(iii) Elements Q and S react together to form an ionic compound. Under normal conditions, which physical state will the compound QS exist in?
(iv) Can Q and S, both be metals? Justify your answer.
(a) (i) C
(ii) C
(iii)D
(b) (i) reduced
(ii) negative
(c) (i) H3O+ ions
Question (2006): (a) Choose the correct answer from the choices A,B,C and D:
(i) The property which is characteristic of an electrovalent compound is that:
A. it is easily vaporized
B. it has a high melting point
C. it is a weak electrolyte,
D. it often exists as a liquid.
(ii) When a metal atom becomes an ion:
A. It loses electrons and is Oxidized
B. It gains electrons and is reduced,
C. It gains electrons and is oxidized,
D. it loses electrons and is reduced.
(b) Identify the following reactions as either oxidation or reduction:
(i) O + 2e- → O2-
(ii) K – e- → K+
(iii) Fe3 + e- → Fe2+
Ans: (a) (i) B
(ii) A
(b) (i) Reduction
(ii) Oxidation
(iii) Reduction
Question (2007): (a) (i) Name the charged particles which attract one another to form electrovalent compounds.
(ii) In the formation of electrovalent compounds, electrons are transferred from one element to another. How are electrons involved in the formation of a covalent compound?
(iii) The electronic configuration of nitrogen is (2, 5). How many electrons in the outer shell of a nitrogen atom are not involved in the formation of a nitrogen molecule?
(iv) In the formation of magnesium chloride (by direct combination between magnesium and chlorine), name the substance that is oxidized and the substance that is reduced.
Ans: (a) (i) The charged particles that form ionic compounds are cations (positive ions) and anions (negative ions).
(ii) In covalent compounds, electrons are shared between atoms.
(iii) Nitrogen’s electronic configuration is (2,5). In an N₂ molecule, each nitrogen atom has 2 non-bonding electrons.
(iv) In the direct combination of magnesium and chlorine, a redox reaction occurs. Magnesium undergoes oxidation by losing electrons to form Mg²⁺ ions. Simultaneously, chlorine is reduced as it gains those electrons to form Cl⁻ ions. This electron transfer is fundamental to the reaction, resulting in the formation of the ionic compound magnesium chloride.
Question (2008): (a) Which of the following is not a common characteristic of an electrovalent compound?
A. High melting point
B. Conducts electricity when molten.
C. Consists of oppositely charged ions
D. Ionizes when dissolved in water.
(b) What are the terms defined below?
(i) A bond formed by a shared pair of electrons, each bonding atom contributing one electron to the pair.
(ii) A bond formed by a shared pair of electrons with both electrons coming from the same atom.
Ans:
(a) (i) D
(b) (i) Covalent bond (ii) Coordinate bond.