Radioactivity is a fascinating and powerful phenomenon where the nucleus of an unstable atom spontaneously emits invisible but energetic particles and radiation to become stable. It was accidentally discovered in 1896 by Henri Becquerel. However, it was the pioneering work of scientists like Marie Curie and Pierre Curie that truly advanced our understanding; they isolated highly radioactive elements like polonium and radium.
This process is entirely natural and originates from within the atom’s nucleus. For a nucleus to be stable, it needs a certain balance between its protons and neutrons. Heavy elements, with their large number of protons and neutrons, often find this balance difficult to maintain, making them radioactive. The three most common types of radiation emitted are named after the first three letters of the Greek alphabet: Alpha (α), Beta (β), and Gamma (γ) rays.
The Three Radiations: Properties and Differences
Alpha Particles (α): These are positively charged particles, identical to the nucleus of a helium atom (2 protons and 2 neutrons). They are heavy, slow-moving, and have the highest ionizing power, meaning they can knock electrons out of atoms very easily. Because of their size and charge, they have very low penetrating power. A sheet of paper or a few centimeters of air can stop them.
Beta Particles (β): These are fast-moving, negatively charged electrons emitted from the nucleus when a neutron transforms into a proton. They are much lighter and smaller than alpha particles. Beta particles have a medium ionizing power and a medium penetrating power—they can pass through paper but are stopped by a thin sheet of aluminum.
Gamma Rays (γ): These are not particles but electromagnetic waves, similar to X-rays but with much higher energy. They have no mass and no charge. They can pass through paper, aluminum, and even lead or thick concrete is needed to reduce their intensity significantly.
Key Concepts and Definitions
Half-Life (T½): This is one of the most important concepts. It is the time taken for half of the radioactive atoms in a sample to disintegrate. It is a fixed value for a particular radioactive element and cannot be changed by any external factors like temperature or pressure. For example, if a substance has a half-life of 5 years, then 1 gram of it will become 0.5 grams in 5 years, 0.25 grams in the next 5 years, and so on.
Radioactive Decay: This is the series of steps a radioactive element goes through, transforming into new elements (called daughter elements) by emitting radiation until a stable, non-radioactive nucleus is finally formed. This entire process is known as a decay series.
Uses of Radioactivity (Applications):
Medical: Radiotherapy uses gamma rays to kill cancerous cells. Radioactive tracers like Iodine-131 are used to diagnose thyroid problems.
Industrial: Gamma rays are used to detect cracks inside metal structures and welds. They are also used to sterilize medical equipment and to control the thickness of sheets in paper and plastic industries.
Archaeological: Carbon-14 dating is used to determine the age of ancient wooden or biological artifacts.
Hazards of Radiations: While useful, radioactivity is dangerous. The radiations can destroy living tissue and cells, cause burns, lead to radiation sickness, and increase the risk of cancer and genetic mutations. Therefore, radioactive materials must be handled with extreme care, using protective lead shields and remote-controlled tools.
In conclusion, radioactivity is a double-edged sword. It is a natural, spontaneous process that provides us with valuable tools in medicine and industry, but it also demands great respect and careful handling due to its potential hazards. Understanding its properties, especially the concept of half-life, is key to using it safely and effectively.
1.Name the three constituents of an atom and state mass and charge of each. How are they distributed in an atom?
Ans: An atom, the basic unit of matter, is built from three core particles. At its heart lies the nucleus, which contains two types of particles: positively charged protons and neutral neutrons, both of which contribute nearly all of the atom’s mass. Swirling rapidly around this dense central core are the electrons. These are tiny, negatively charged particles with a mass so small it is generally considered insignificant when describing the atom’s overall weight. The number of protons defines the element’s identity, while the arrangement of electrons determines its chemical behavior.
2.Define the terms:
(a) atomic number, and
(b) mass number.
Ans: Atomic Number (Z):
The atomic number, written as Z, is the count of protons in an atom’s nucleus. It is the element’s unique identity card. For a neutral atom, it also tells you the number of electrons. This number defines the element and its position on the periodic table.
Mass Number (A):
The mass number, written as A, is the total number of protons and neutrons in the atom’s nucleus. It gives a rough estimate of the atom’s mass. You can find the number of neutrons by subtracting the atomic number from the mass number: Neutrons = A – Z.
3.What is nucleus of an atom? Compare its size with that of the atom. Name its constituents. How is the number of these constituents determined by the atomic number and mass number of the atom?
Ans: The Nucleus of an Atom
The nucleus is the dense, central part of an atom, containing most of its mass. It is positively charged and consists of two types of particles: protons and neutrons, collectively called nucleons.
Size Comparison:
The size (diameter) of the nucleus is extremely small compared to the entire atom.
Nucleus size: approximately 10⁻¹⁵ m
Atom size: approximately 10⁻¹⁰ m
Thus, the nucleus is about 1/100,000th (10⁻⁵) the diameter of the atom. If an atom were the size of a football stadium, the nucleus would be only about the size of a pea at the center.
Constituents of the Nucleus:
Protons: Positively charged particles.
Neutrons: Neutral particles (no electric charge).
Determining the Number of Constituents:
Atomic Number (Z):
It is equal to the number of protons in the nucleus.
Number of protons=Z
Mass Number (A):
It is the total number of protons and neutrons (nucleons) in the nucleus.
Number of neutrons=A−Z
So, for any atom:
Protons = Atomic Number (Z)
Neutrons = Mass Number (A) – Atomic Number (Z)
4.State the atomic number and mass number of 211 Na and draw its atomic model.
(Note: The notation appears unusual; likely refers to sodium with mass number 23 and atomic number 11.)
Ans:
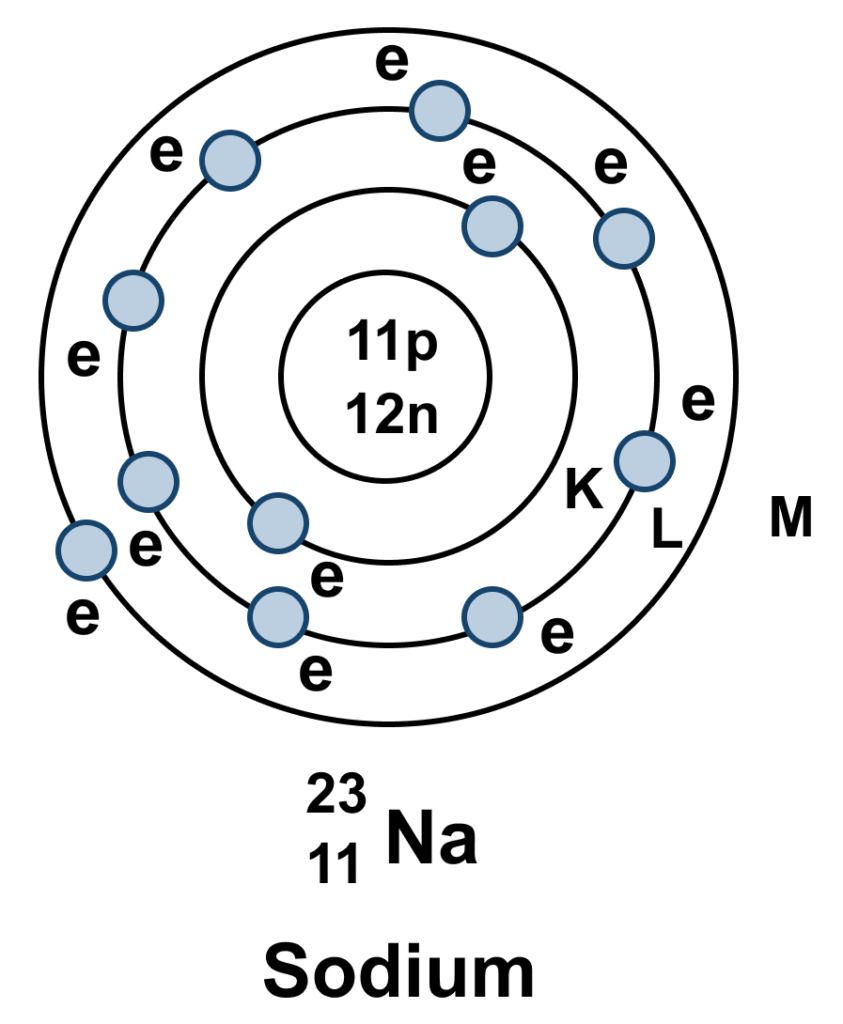
the atomic structure of Sodium (Na):
Symbol: Na
Atomic Number (Z): 11
Mass Number (A): 23
Protons: 11
Neutrons: 12
Electrons: 11
Bohr Model Arrangement:
The 11 electrons are arranged in three energy levels or shells around the nucleus.
First Shell (K): Holds 2 electrons.
Second Shell (L): Holds 8 electrons.
Third Shell (M): Holds 1 electron.
A simple sketch would show a central nucleus labeled with 11 protons and 12 neutrons, surrounded by three circles. The inner circle has 2 dots, the middle has 8, and the outer circle has 1 dot representing the electrons.
5.What are isotopes? Give one example.
Ans: Isotopes are atoms of the same chemical element that have the same number of protons (and hence the same atomic number) but different numbers of neutrons. This results in different mass numbers for the atoms.
Example:
A common example is Carbon, which has three main isotopes:
Carbon-12 (6 protons, 6 neutrons)
Carbon-13 (6 protons, 7 neutrons)
Carbon-14 (6 protons, 8 neutrons)
All carbon isotopes have 6 protons, but they differ in their number of neutrons.
6.What are isobars? Give one example.
Ans: Isobars are atoms from different elements that coincidentally share the same mass number, meaning the total number of their protons and neutrons is identical. However, their individual composition differs; one element will have more protons and fewer neutrons, while the other has fewer protons and more neutrons. A classic example is Argon-40, which has 18 protons and 22 neutrons, and Calcium-40, with 20 protons and 20 neutrons. Despite both having a mass number of 40, their differing proton numbers place them in separate positions on the periodic table, making them distinct chemical elements.
7.Name the atoms of a substance having same atomic number, but different mass numbers. Give one example of such a substance. How do the structures of such atoms differ?
Ans: Atoms of a substance having the same atomic number but different mass numbers are called isotopes.
Example: Hydrogen has three isotopes:
Protium (¹H) – Mass number 1
Deuterium (²H) – Mass number 2
Tritium (³H) – Mass number 3
Difference in structure:
All three have the same atomic number (1), meaning they have 1 proton in the nucleus and 1 electron revolving around it.
Protium has 0 neutrons,
Deuterium has 1 neutron,
Tritium has 2 neutrons.
8.What is meant by radioactivity? Name two radioactive substances.
Ans: Radioactivity is the spontaneous disintegration of the nuclei of certain unstable elements, leading to the emission of radiation in the form of alpha particles, beta particles, or gamma rays.
Two radioactive substances are:
Uranium-238
Radium-226
9.A radioactive substance is oxidised. What changes would you expect to take place in the nature of radioactivity? Explain your answer.
Ans. When a radioactive substance undergoes a chemical change such as oxidation, no change takes place in its radioactivity.
This is because radioactivity is a nuclear phenomenon, dependent on the nucleus of the atom. Chemical reactions like oxidation involve only the electrons surrounding the nucleus and do not affect the nucleus itself. Therefore, the type of radiation emitted, the half-life, and the intensity of radioactivity remain completely unchanged.
For example, if radioactive iodine is oxidized to form iodine molecules or other compounds, it will continue to decay at the same rate and emit the same radiation as before the chemical reaction.
10.A radioactive source emits three types of radiations.
(a) Name the three radiations.
(b) Name the radiations which are deflected by the electric field.
(c) Name the radiation which is most penetrating.
(d) Name the radiation which travels with the speed of light.
(e) Name the radiation which has the highest ionising power.
(f) Name the radiation consisting of the same kind of particles as the cathode rays.
Ans: (a) Name the three radiations.
The three types of radiations emitted by a radioactive source are Alpha (α) particles, Beta (β) particles, and Gamma (γ) rays.
(b) Name the radiations which are deflected by the electric field.
Alpha and Beta radiations are deflected by an electric field. Alpha particles, being positively charged, are deflected towards the negative plate. Beta particles, which are negatively charged, are deflected towards the positive plate.
(c) Name the radiation which is most penetrating.
Gamma radiation is the most penetrating. It can pass through several centimeters of lead and requires very dense materials, like thick concrete or lead, to be significantly reduced.
(d) Name the radiation which travels with the speed of light.
Gamma radiation travels with the speed of light. This is because gamma rays are a form of electromagnetic radiation, just like light, but with much higher energy.
(e) Name the radiation which has the highest ionising power.
Alpha radiation has the highest ionising power. Because alpha particles are relatively heavy and carry a double positive charge, they interact strongly with matter, knocking electrons out of atoms and creating a large number of ions over a short distance.
(f) Name the radiation consisting of the same kind of particles as the cathode rays.
Beta radiation consists of the same kind of particles as cathode rays. Both are streams of high-speed electrons.
11.A radioactive source emits three types of radiations.
(a) Name the radiation of zero mass.
(b) Name the radiation which has the lowest ionising power.
(c) Name the radiation which has the lowest penetrating power.
(d) Give the charge and mass of particles composing the radiation in part (c).
(e) When the particle referred to in part (c) becomes neutral, it is found to be the atom of a rare gas. Name this rare gas and draw a model of its neutral atom.
(f) From which part of the atom do these radiations come?
Ans:(a) Name the radiation of zero mass.
The radiation with zero mass is the Gamma (γ) radiation. It is an electromagnetic wave and not composed of particles with mass.
(b) Name the radiation which has the lowest ionising power.
Gamma (γ) radiation has the lowest ionising power. Because it has no charge and high penetration, it interacts less with matter and causes fewer ionisations per unit path length compared to other radiations.
(c) Name the radiation which has the lowest penetrating power.
Alpha (α) radiation has the lowest penetrating power. It can be stopped by a sheet of paper or a few centimetres of air.
(d) Give the charge and mass of particles composing the radiation in part (c).
The radiation in part (c) is Alpha (α) radiation.
Charge: +2 units (equivalent to the charge of two protons)
Mass: 4 atomic mass units (a.m.u.). It is identical to a helium nucleus (He2+ ).
(e) When the particle referred to in part (c) becomes neutral, it is found to be the atom of a rare gas. Name this rare gas and draw a model of its neutral atom.
The rare gas is Helium.
A model of its neutral atom can be drawn as follows:
A central nucleus containing 2 protons and 2 neutrons.
Two electrons orbiting the nucleus in a shell (or energy level).
text
(Nucleus: 2p + 2n)
/ \
/ \
(e-) o o (e-)
\ /
\ /
(Electron Shell)
Explanation: The alpha particle (He²⁺) is a helium nucleus. When it gains two electrons from the surrounding environment, it becomes a neutral helium atom (He).
(f) From which part of the atom do these radiations come?
These radiations come from the nucleus of the atom. Radioactive decay is a process that involves the unstable nucleus rearranging itself to become more stable, and in doing so, it emits these radiations.
12. The diagram in Fig. 12.7 shows a radioactive source S placed in a thick lead walled container. The radiations given out are allowed to pass through a magnetic field. The magnetic field (shown as x) acts perpendicular to the plane of paper inwards. Arrows show the paths of the radiations A, B and C.
(a) Name the radiations labelled A, B and C.
(b) Explain clearly how you used the diagram to arrive at the answer in part (a).
Ans: (a) Name the radiations labelled A, B and C.
A: Alpha radiation (α)
B: Gamma radiation (γ)
C: Beta radiation (β⁻)
(b) Explain clearly how you used the diagram to arrive at the answer in part (a).
The diagram shows the effect of a magnetic field (acting into the page) on the three radiations.
Radiation A is deflected slightly to the left. This indicates it is a positively charged particle. Since it is less deflected than Radiation C, it must be the heavier alpha particle.
Radiation B shows no deflection and travels straight. This means it has no electric charge, identifying it as neutral gamma radiation.
Radiation C is deflected strongly to the right. This indicates it is a charged particle. The direction of deflection is opposite to Radiation A, so it is negatively charged, identifying it as the light beta particle.
13. Fig. 12.8 shows a mixed source R of alpha and beta particles in a thick lead walled container. The particles pass through a magnetic field in a direction perpendicular to the plane of paper inwards as shown by x.
(a) Show in the diagram how the particles get affected.
(b) Name the law used in part (a).
[Hint: alpha particles will deflect to the left while beta particles to the right]
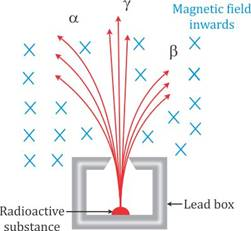
Ans:(a) In the magnetic field (directed into the plane of the paper):
Alpha particles will deflect towards the left.
Beta particles will deflect towards the right.
(b) The law used is Fleming’s Left-Hand Rule.
14. Fig. 12.9 shows a radioactive source S in a thick lead walled container having a narrow opening. The radiations pass through an electric field between the plates A and B.
(a) Complete the diagram to show the paths of α, β and γ radiations.
(b) Why is the source S kept in a thick lead walled container with a narrow opening?
[Hint: α radiations will deflect towards the negative plate, β radiations towards the positive plate and γ radiations remain undeflected]
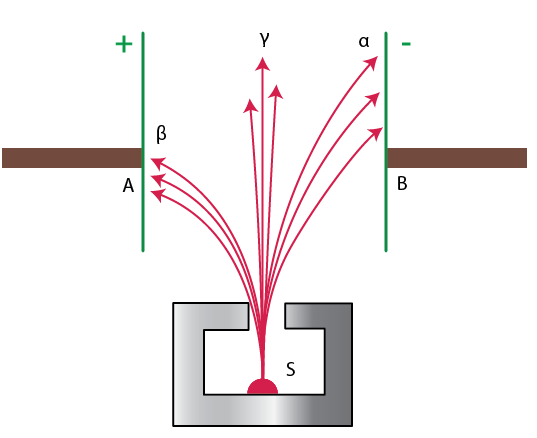
Ans: (a) In the diagram:
α radiations (positively charged) will bend towards the negatively charged plate (B).
β radiations (negatively charged) will bend towards the positively charged plate (A).
γ radiations (neutral) will go straight without any deflection.
(b) The radioactive source is kept in a thick lead-walled container with a narrow opening to:
Block most of the harmful radiations.
Allow only a narrow beam of radiations to come out for the experiment, ensuring safety and clear observation.
15. Explain why alpha and beta particles are deflected in an electric or a magnetic field, but gamma rays are not deflected in such a field.
[Hint: alpha and beta particles are charged, but gamma rays are uncharged]
Ans: Alpha and beta particles are deflected in electric or magnetic fields because they carry an electric charge (alpha particles are positively charged, and beta particles are negatively charged). These charged particles experience a force when moving through such fields, causing their paths to bend.
Gamma rays, however, are not deflected because they are electromagnetic radiation and carry no electric charge. Since they are uncharged, electric and magnetic fields exert no force on them, and they continue to travel in a straight line.
16. Is it possible to deflect γ radiations in a way similar to α and β-particles, using the electric or magnetic field? Give reason.
Ans: γ-rays are electromagnetic radiation (high-energy photons) and carry no electric charge. Only charged particles like α-particles (positive) and β-particles (negative) experience deflection in such fields. Since γ-rays are neutral, they pass through undeviated.
17. State following four properties each of α, β and γ radiations:
(a) nature,
(b) charge,
(c) mass, and
(d) effect of electric field.
Ans: (a) Nature
α (Alpha): Helium nucleus (stream of positive particles)
β (Beta): High-speed electrons
γ (Gamma): Electromagnetic radiation (like light)
(b) Charge
α: Positive charge (+2)
β: Negative charge (-1)
γ: No charge (neutral)
(c) Mass
α: Relatively large mass (4 u)
β: Very small mass (like an electron)
γ: No mass
(d) Effect of Electric Field
α: Deflected towards negative plate
β: Deflected strongly towards positive plate
γ: Not deflected (goes straight)
18. Arrange the α, β and γ radiations in ascending order of their
(i) ionising power, and
(ii) penetrating power.
Ans: (i) γ < β < α (ii) α < β < γ
19. State the speed of each of α, β and γ radiations.
Ans:107 m s−1
20. (a) What is the composition of α, β and γ radiations?
(b) Which one α, β or γ radiation has the least penetrating power?
Ans: (a) Composition:
α-radiation: Helium nucleus (2 protons + 2 neutrons)
β-radiation: High-speed electron
γ-radiation: High-energy electromagnetic wave (photon)
(b) Least Penetrating Power:
α-radiation has the least penetrating power. It can be stopped by a sheet of paper or a few centimeters of air.
21. How are γ radiations produced? Mention two common properties of the gamma radiations and visible light.
Ans:
Gamma (γ) radiations are produced during the rearrangement of an atomic nucleus. This happens when a nucleus that is in an unstable, high-energy state transitions to a more stable, lower-energy state. The excess energy is emitted in the form of a gamma ray photon. This frequently occurs as a follow-up process right after other nuclear events, such as radioactive alpha or beta decay, or during nuclear reactions like fusion or fission. Essentially, gamma rays are the means by which an excited nucleus releases its leftover energy to achieve stability.
Two common properties of the gamma radiations and visible light.
Despite their vast difference in energy, gamma rays and visible light share two fundamental properties:
They are both electromagnetic waves. This means they both travel at the same incredible speed of light (approximately 3 × 10⁸ m/s) in a vacuum and consist of oscillating electric and magnetic fields.
They exhibit wave-like phenomena such as reflection, refraction, and diffraction. Just like visible light can be reflected by a mirror or bent by a lens, gamma rays also obey these principles of wave optics, although their extremely short wavelength makes the effects much harder to observe without specialized equipment.
22. An α-particle captures
(i) one electron,
(ii) two electrons.
In each case, what does it change to?
Ans. When an alpha-particle, which is essentially a helium nucleus, captures electrons, it starts to become a neutral atom by gaining the electrons it was missing. Here’s what happens in each case:
(i) Captures one electron:
If it captures just one electron, which carries a charge of -1, its overall charge becomes +1. At this stage, it is no longer a neutral atom, but a positive ion. Specifically, it changes into a Helium ion (He⁺). This is also known as a singly-ionized helium atom.
(ii) Captures two electrons:
If the alpha-particle captures a second electron, its total charge becomes neutral (+2 from the protons and -2 from the electrons equals 0). With a full complement of two electrons in its orbit, it completes its structure and changes into a stable, neutral Helium atom (He).
23. ‘Radioactivity is a nuclear phenomenon’. Comment on this statement.
Ans: This statement is correct because the process of radioactivity originates entirely within the nucleus of an atom and is independent of the external environment or the chemical bonds the atom may form. The stability of a nucleus depends on the delicate balance between the strong nuclear force (which holds protons and neutrons together) and the electrostatic repulsion between the positively charged protons.
In many heavy elements, like Uranium or Radium, the nucleus itself is inherently unstable due to an excess of either protons or neutrons. To achieve a more stable, lower-energy state, these unstable nuclei undergo a spontaneous disintegration or transformation. This process of the nucleus changing its composition to become more stable is what we call radioactivity. During this transformation, the nucleus emits energy and particles such as:
Alpha particles (which are helium nuclei, consisting of 2 protons and 2 neutrons).
Beta particles (which are high-speed electrons or positrons).
Gamma rays (which are high-energy electromagnetic photons).
Since the protons and neutrons are constituents of the nucleus, and the emitted particles and energy come from within this nucleus, the entire process is intrinsic to the nucleus. This is why changing the atom’s physical state (e.g., from solid to liquid) or its chemical composition (e.g., forming a compound) has no effect on its radioactive properties. The rate of decay remains constant because the cause of the instability lies purely in the nuclear configuration, not in the arrangement of the atom’s electrons. Therefore, radioactivity is rightly classified as a purely nuclear phenomenon.
24. What kind of change takes place in a nucleus when a β-particle is emitted? Express it by an equation. State whether
(a) atomic number, and
(b) mass number are conserved in a radioactive β-decay?
Ans: When a beta particle (β-particle) is emitted from a nucleus, a neutron is transformed into a proton, and the process also produces an electron (the beta particle) and an antineutrino.
This change increases the atomic number by 1 but leaves the mass number unchanged.
The general equation for β⁻ decay is:
ZAX→Z+1AY+−10e+νˉ
Where:
ZAX is the parent nucleus,
Z+1AYis the daughter nucleus,−10e is the beta particle (electron), is the antineutrino.
(a) Atomic number: Not conserved — it increases by 1.
(b) Mass number: Conserved — remains the same.
25. A certain radioactive nucleus emits a particle that leaves its mass unchanged, but increases its atomic number by one. Identify the particle and write its symbol.
Ans. beta particle, β−
Ans:
The particle described, which increases the atomic number by one without changing the mass number, is the beta particle.
Its symbol is β⁻.
Explanation:
This happens during a process called beta-minus (β⁻) decay. Inside the nucleus, a neutron is transformed into a proton. Since a proton has an atomic number one higher than a neutron, this conversion causes the atomic number (Z) to increase by one. The mass number (A), which is the total count of protons and neutrons, remains unchanged because the total number of nucleons stays the same—one neutron is simply replaced by one proton. The beta particle (a high-energy electron) and an antineutrino are emitted in this process to carry away energy and conserve other properties.
26. What happens to the (i) atomic number, (ii) mass number of an element when (a) an α-particle, (b) a β-particle, and (c) γ radiation, is emitted?
Ans:(a) When an α-particle is emitted:
(i) Atomic Number: Decreases by 2.
(ii) Mass Number: Decreases by 4.
Reason: An alpha particle is a helium nucleus (2 protons and 2 neutrons). Losing it reduces both the proton count (atomic number) and the total nucleons (mass number).
(b) When a β-particle is emitted:
(i) Atomic Number: Increases by 1.
(ii) Mass Number: Remains the same.
Reason: A beta particle is an electron ejected from a neutron. This changes a neutron into a proton, increasing the proton count by one. The total number of protons and neutrons (mass number) stays unchanged.
(c) When γ radiation is emitted:
(i) Atomic Number: Remains the same.
(ii) Mass Number: Remains the same.
Reason: Gamma rays are pure energy (high-energy photons). They are emitted to release excess energy from the nucleus, but the number of protons and neutrons does not change.
27. What happens to the position of an element in the periodic table when it emits (a) an α-particle, (b) a β-particle and (c) γ radiation? Give reason for your answer.
Ans: (a) Emission of an α-particle:
Change in Position: The element moves two places to the left in the periodic table.
Reason: An alpha (α) particle is identical to a helium nucleus, consisting of 2 protons and 2 neutrons. When an atom emits an α-particle, its atomic number (Z) decreases by 2 (because it loses 2 protons), and its mass number (A) decreases by 4 (because it loses 2 protons and 2 neutrons). Since the atomic number defines an element’s identity, a decrease of 2 means it transforms into a new element that is two boxes to the left on the periodic table. For example, if Radium (Z=88) emits an alpha particle, it becomes Radon (Z=86).
(b) Emission of a β-particle:
Change in Position: The element moves one place to the right in the periodic table.
Reason: A beta (β-) particle is a high-energy electron. It is emitted when a neutron in the nucleus transforms into a proton. This process increases the atomic number (Z) by 1 (as a new proton is created), while the mass number remains almost the same. This increase of one in the atomic number means the element transforms into the next element to its right. For instance, if Carbon-14 (Z=6) undergoes beta decay, one of its neutrons becomes a proton, changing it into Nitrogen-14 (Z=7).
(c) Emission of γ radiation:
Change in Position: The element remains in the same position in the periodic table.
Reason: Gamma (γ) rays are high-energy electromagnetic radiation, similar to X-rays but more powerful. The emission of γ-radiation involves the nucleus losing excess energy to become more stable. Crucially, this process does not change the atomic number (Z) or the mass number (A) of the nucleus. It only changes the energy state of the nucleus. Since the atomic number remains the same, the identity of the element is unchanged, and its position in the periodic table stays fixed.
28. What changes occur in the nucleus of a radioactive element when it emits (a) an alpha particle, (b) a beta particle, (c) gamma radiation? Give one example, in each case (a) and (b) in support of your answer.
Ans:
(a) Emission of an Alpha Particle (α-particle)
Change in Nucleus: The atomic number (Z) decreases by 2, and the mass number (A) decreases by 4.
New Atomic Number (Z) = Z – 2
New Mass Number (A) = A – 4
Reason: The nucleus loses a cluster of 2 protons and 2 neutrons.
Example: When Uranium-238 emits an alpha particle, it becomes Thorium-234.
92
238U→Th+24α92
(b) Emission of a Beta Particle (β-particle)
Change in Nucleus: The atomic number (Z) increases by 1, but the mass number (A) remains the same.
New Atomic Number (Z) = Z + 1
New Mass Number (A) = A
Reason: A neutron inside the nucleus is transformed into a proton and an electron. The electron is emitted as the beta particle.
Example: When Carbon-14 emits a beta particle, it becomes Nitrogen-14.
614C→714N+−10β
(c) Emission of Gamma Radiation (γ-rays)
Change in Nucleus: There is no change in the atomic number (Z) or the mass number (A).
Reason: The nucleus releases excess energy in the form of electromagnetic radiation (gamma rays) to become more stable. This often happens after a previous alpha or beta decay has left the nucleus in an excited, high-energy state.
29.(a) An atomic nucleus A is composed of 84 protons and 128 neutrons. The nucleus A emits an α-particle and is transformed into a nucleus B. What is the composition of B?
(b) The nucleus B emits a β-particle and is transformed into a nucleus C. What is the composition of C?
(c) What is the mass number of the nucleus A?
(d) Does the composition of nucleus C change if it emits γ radiation?
Ans: (a) Composition of nucleus B:
Original nucleus A: 84 protons, 128 neutrons.
An α-particle has 2 protons and 2 neutrons.
After α-emission:
Protons = 84 − 2 = 82
Neutrons = 128 − 2 = 126
So, B has 82 protons and 126 neutrons.
(b) Composition of nucleus C:
Nucleus B: 82 protons, 126 neutrons.
A β-particle emission means a neutron changes into a proton.
After β-emission:
Protons = 82 + 1 = 83
Neutrons = 126 − 1 = 125
So, C has 83 protons and 125 neutrons.
(c) Mass number of nucleus A:
Mass number = protons + neutrons = 84 + 128 = 212.
(d) Effect of γ radiation:
γ radiation does not change the proton or neutron number.
So, composition of nucleus C remains the same if it emits γ radiation.
30. A certain nucleus A (mass number 238 and atomic number 92) is radioactive and becomes a nucleus B (mass number 234 and atomic number 90) by the emission of a particle.
(a) Name the particle emitted.
(b) Explain how you arrived at your answer.
(c) State the change in the form of a reaction.
Ans:
When a radioactive nucleus emits an alpha particle (α-particle):
Its mass number decreases by 4
Its atomic number decreases by 2
In the given reaction:
92238A→90234B+24HeX
The α-particle emitted is a helium nucleus (24HeX 2).
31. State whether the following nuclear disintegrations are allowed or not (star indicates an excited state). Give reason if it is not allowed.
(a) AZX∗→AZX+γ
(b) AZX→A−2Z−4X+42He
Ans: Based on the provided question, here is the answer:
(a) ZAX∗→ZAX+γ
Answer: Allowed.
Reason: This reaction represents gamma decay. A nucleus in an excited state (X∗ ) releases its excess energy by emitting a gamma-ray photon (γ). This process conserves both mass number (A) and atomic number (Z), which is required.
(b) ZAX→Z−4A−2X+24He
Answer: Not Allowed.
Reason: The emitted particle is an alpha particle (24 He), which should cause the daughter nucleus to have an atomic number of Z−2 and a mass number of
A−4. In this reaction, the atomic number is given as Z−4, which violates the conservation of atomic number (and charge). The correct disintegration should be:
ZAX→Z−2A−4X+24He
32. A nucleus 1124Na is β-radioactive.
(a) What are the numbers 24 and 11 called?
(b) Write the equation representing β-decay.
(c) What general name is given to the product nucleus with respect to 1124Na
Ans: (a) The number 24 is the mass number, and the number 11 is the atomic number.
(b) The equation representing the β⁻ decay is:
1124Na → 1224Mg +−10β+ν‾e
(c) The general name given to the product nucleus with respect to 1124 Na is the daughter nucleus.
33. A nucleus of stable phosphorus has 15 protons and 16 neutrons.
(a) What is its atomic number and mass number?
(b) The nucleus of radio phosphorous has one neutron more than the stable nucleus. What will be its atomic number and mass number?
(c) What will be the atomic number and mass number of the new nucleus formed by the decay of a β-particle by the radio phosphorous in part (b)?
Ans:
(a) Stable Phosphorus Nucleus
Atomic number (Z) = Number of protons = 15
Mass number (A) = Protons + Neutrons = 15 + 16 = 31
(b) Radio Phosphorus Nucleus
Neutrons = 16 + 1 = 17
Atomic number (Z) = Protons = 15 (unchanged)
Mass number (A) = 15 + 17 = 32
(c) After β⁻ Decay
In β⁻ decay, a neutron changes into a proton, so:
Atomic number (Z) = 15 + 1 = 16
Mass number (A) = 32 (remains same)
The new element is Sulfur-32.
34. An element P disintegrates by α emission and the new element suffers two further disintegrations, both by β emission, to form an element Q. Explain the fact that P and Q are isotopes.
Ans: Elements P and Q are isotopes because they have the same atomic number but different mass numbers. Here’s why:
α Emission (from P): When element P emits an alpha particle (He²⁺ nucleus, 2 protons and 2 neutrons), its atomic number decreases by 2 and its mass number decreases by 4, forming a new element.
First β Emission: A beta particle is a high-energy electron. Its emission from a nucleus converts a neutron into a proton. This increases the atomic number by 1, creating yet another element, but the mass number stays the same.
Second β Emission: Another beta emission occurs, converting another neutron into a proton. This increases the atomic number by 1 again.
After these two beta decays, the atomic number has increased by a total of 2 from the element formed right after the alpha decay.
The Key Point: The initial alpha emission decreased the atomic number by 2. The two subsequent beta emissions increased it by 2. The net change in the atomic number is zero.
Therefore, the final element Q has the same atomic number as the original element P (defining them as the same element), but a lower mass number (due to the initial loss of 4 nucleons from the alpha particle). This fits the definition of isotopes: same atomic number, different mass number.
35. A nucleus ZAX emits 2 α particles and 1 β particle to form a nucleus 89228R
Find the atomic number and mass number of X.
Ans:
Step 1: Initial and final nucleus
We are told:
Final nucleus is 89228R
Initial nucleus is ZAX, which emits 2 α particles and 1 β⁻ particle.
Step 2: Effect of α decay
An α particle is 24 He.
So 2 α particles emitted means:
Mass number decreases by 4×2=8
Atomic number decreases by 2×2=4
Step 3: Effect of β⁻ decay
A β⁻ particle is −10e, but in nuclear terms:
n→p+e − + νˉ e→ atomic number increases by 1, mass number unchanged.
So 1 β⁻ emission means:
Mass number: no change
Atomic number: increases by 1
Step 4: Work backwards from final to initial
Final: 89
228R
We reverse the emissions to find initial nucleus.
Reversing means:
Reverse α emission: add 8 to mass number, add 4 to atomic number
Reverse β⁻ emission: subtract 1 from atomic number (since β⁻ emission increased Z by 1, reversing decreases Z by 1)
But careful: The nucleus first emitted α, α, β (or in any order — but the net effect is the same: ΔA = –8, ΔZ = –4 + 1 = –3).
So from final to initial:
Initial Z = Final Z + 3 = 89 + 3 = 92
Initial A = Final A + 8 = 228 + 8 = 236
Step 5: Conclusion
Initial nucleus: 92236X
Atomic number = 92
Mass number = 236
36. Complete the following sentences:
(a) The mass number and atomic number of an element are not changed when it emits ………………
(b) The atomic number of a radioactive element is not changed when it emits ………………
(c) During the emission of a beta particle, the ………… number remains same.
Ans:
36. Complete the following sentences:
(a) The mass number and atomic number of an element are not changed when it emits gamma radiation.
(b) The atomic number of a radioactive element is not changed when it emits gamma rays.
(c) During the emission of a beta particle, the mass number remains same.
37. Complete the following nuclear changes:
(a) 92P→Q+??
(b) 23892U→23490Th+….+energy
(c) 23892P→Q→R→S
(d) 42X1→X2→X3
(e) X→X1→X2→??X3
Ans:
(a) 92238U →90234Th+24α
(b) 92238U →90234Th+24α+energy
(c) 92238U →α90234Th →β−91234Pa →β−92234U92
(d) 92238U →α90234Th →β−91234Pa →β−92234U92
(e) 90232Th →α88228Ra →β−89228Ac →β−90228Th
38. What are radio isotopes? Give one example of a radio isotope. State one use of radio isotopes.
Ans: Radioisotopes are unstable isotopes of an element that emit radiation in the form of alpha, beta, or gamma rays as they decay to become more stable. They are the radioactive forms of elements.
Example: A common example is Cobalt-60 (Co-60).
One Use: Radioisotopes are used in medicine for radiotherapy. Specifically, Cobalt-60 is used to treat cancer, as its gamma rays can be targeted to destroy malignant tumors.
39. Why are the alpha particles not used in radio therapy?
Ans: Alpha particles are not used in radiotherapy primarily because they have a very short range in tissue (only a few cell diameters) and are strongly ionizing. This means they cannot penetrate the skin to reach internal tumors, and if used, they would cause severe damage to healthy surface tissues without effectively treating deeper cancerous growths.
40. Why do we usually use isotopes emitting gamma radiations as radioactive tracers in medical science?
Ans: Gamma-emitting isotopes are preferred as radioactive tracers in medical science primarily because gamma rays have high penetration power but low ionization power.
This combination is crucial because:
High penetration allows gamma rays to exit the body and be detected by external scanners (like a Gamma Camera or PET scanner), enabling clear imaging of the tracer’s location inside the body.
Low ionization causes significantly less damage to biological tissues compared to alpha or beta radiation, making the procedure safer for the patient.
Essentially, they provide a safe and effective way to “see” inside the body without causing substantial harm.
41. When does the nucleus of an atom become radioactive?
Ans: The nucleus of an atom becomes radioactive when it is unstable. This instability arises because the forces within the nucleus (specifically, the strong nuclear force that holds protons and neutrons together) are unbalanced and cannot overcome the natural repulsion between the positively charged protons.
An atom seeks a stable, balanced state. A nucleus is often unstable if:
It has too many or too few neutrons compared to its number of protons. This disrupts the delicate balance between the strong force and the electromagnetic repulsion.
It is simply too heavy. Elements with an atomic number higher than 82 (Lead) are almost always radioactive because their large size makes them inherently unstable.
To achieve stability, the unstable (radioactive) nucleus must transform itself. It does this by spontaneously emitting energy and particles in the form of radiation—such as alpha particles, beta particles, or gamma rays. This process of transformation is known as radioactive decay, and it continues until the nucleus reaches a stable configuration.
42. Which of the following is the radio isotope in each pair (a), (b) and (c)?
(a) 126C,146C
(b) 3015P,3215P
(c) 3919K,4019K
Give reason for your answer.
Ans: (a) Radioisotope: ₆C¹⁴ (Carbon-14)
Reason: C-12 is stable with 6 protons and 6 neutrons. C-14 has 2 extra neutrons, making its nucleus unstable. It decays by emitting beta particles to achieve stability.
(b) Radioisotope: ₁₅P³² (Phosphorus-32)
Reason: P-30 and P-32 are both unstable, but P-32 is the well-known radioisotope. With 17 neutrons (more than the stable P-31), it undergoes beta decay to form sulfur-32.
(c) Radioisotope: ₁₉K⁴⁰ (Potassium-40)
Reason: K-39 is stable, while K-40 has an unstable nucleus due to its higher neutron count. It decays slowly through beta decay or electron capture, making it naturally radioactive.
43. State the medical use of radioactivity.
Ans: Medical Uses of Radioactivity:
Diagnosis (Imaging): Radioactive tracers (like Technetium-99m) are introduced into the body. A special camera then detects the radiation they emit to create images of organs like the thyroid, bones, heart, and brain, helping to identify tumours, blockages, or other functional problems.
Cancer Treatment (Radiotherapy): High-energy radiation from sources like Cobalt-60 is focused on cancerous tumours. This radiation kills the rapidly dividing cancer cells or damages their DNA, stopping them from multiplying.
Sterilisation: Gamma rays from a radioactive source like Cobalt-60 are used to sterilise medical equipment (e.g., syringes, surgical gloves) and dressings. The radiation kills bacteria and other microorganisms without damaging the materials.
44. Arrange the α, β and γ radiation in ascending order of their biological damage. Give reason.
Ans: Ascending order of biological damage:
α < β < γ
Reason:
Alpha (α) particles are heavy and double-charged, so they interact strongly with matter and lose energy quickly, causing intense damage but only over a very short range (can be stopped by skin or paper). Beta (β) particles are lighter and penetrate slightly deeper than alpha, causing moderate damage. Gamma (γ) rays are highly penetrating electromagnetic radiation; they pass through the body easily, causing less intense but widespread damage to cells and DNA, making them the most dangerous for internal biological damage.
45. Name two main sources of nuclear radiations. How are the nuclear radiations harmful?
Ans: Two main sources of nuclear radiation are:
Natural Sources: These are always around us. A major example is Radon-222 gas, which can seep from the ground into buildings. Another common source is rocks and soil that contain trace amounts of radioactive elements like Uranium. Even our food and the sun (cosmic rays) expose us to low levels of natural radiation.
Man-Made Sources: These are created for specific purposes. The most significant are the radioactive materials used in nuclear power plants and nuclear weapons. Other important sources are those used in medicine, such as radioactive tracers for diagnosis (like Technetium-99m) and for cancer treatment (like Cobalt-60).
Harmful effects of nuclear radiations:
Nuclear radiation is harmful because it carries a lot of energy that can damage or destroy living cells. This happens in two main ways:
Somatic Damage (Damage to the Body): This is the immediate injury to a person exposed to high levels of radiation. It can cause radiation sickness, with symptoms like nausea, hair loss, and burns. In the long term, it severely damages the DNA inside cells, which can lead to various cancers like leukemia or thyroid cancer.
Genetic Damage (Damage to Future Generations): If the radiation damages the DNA in a person’s reproductive cells (sperm or egg), it can cause mutations. These genetic changes can then be passed on to their children, potentially leading to birth defects and hereditary diseases in future generations.
46. State two safety measures to be taken while establishing a nuclear power plant.
Ans: Two important safety measures for a nuclear power plant are:
Constructing a Robust Containment Structure: Building a thick, heavily reinforced concrete and steel dome around the reactor. This structure is designed to contain any accidental release of radioactive materials, preventing them from escaping into the environment.
Implementing Multiple Cooling Systems: Ensuring there are several independent and redundant cooling systems. These systems are vital to remove excess heat from the reactor core, even during a shutdown or power failure, to prevent the fuel from overheating and melting.
47. What is meant by nuclear waste? State one way for the safe disposal of nuclear waste.
Ans: Nuclear waste is the radioactive and hazardous material left over after nuclear reactions, such as those in nuclear power plants or nuclear weapons production.
One way for safe disposal is deep geological disposal. This involves sealing the nuclear waste in special containers and burying it deep underground in stable rock formations to isolate it from the environment for thousands of years.
48. State three safety precautions that you would take while handling the radioactive substances.
Ans: Here are three safety precautions for handling radioactive substances:
Use Protective Shielding: Always handle radioactive materials behind appropriate shields (e.g., lead or concrete barriers) that are sufficient to block or significantly reduce the radiation exposure.
Maintain Distance: Use long-handled tools like tongs or manipulators to handle substances. Maximizing the distance between yourself and the radioactive source greatly reduces your exposure.
Limit Exposure Time: Plan and practice procedures in advance to minimize the amount of time you spend working directly with or near radioactive materials.
49. Why should a radioactive substance not be touched by hand?
Ans: A radioactive substance should not be touched by hand for two main reasons:
External Hazard: The radiation it emits (like alpha, beta, and gamma rays) can pass through the skin and damage living tissues and cells. This can cause severe burns, increase the risk of cancer, and potentially lead to radiation sickness.
Internal Contamination: If even a tiny, invisible amount of the substance is accidentally transferred from the hand to the mouth (through eating or drinking), it can get inside the body. Once inside, it continuously irradiates internal organs, causing far more serious and long-term damage than external exposure.
Essentially, touching it poses both an immediate external radiation risk and a serious risk of permanent internal contamination.
50. What do you mean by background radiations? Name its two sources. Is it possible for us to keep ourselves away from it?
Ans: Background radiation is the low-level ionizing radiation always present in our environment.
Two sources are:
Natural sources – e.g., radon gas from the ground, cosmic rays from space.
Man-made sources – e.g., medical X-rays, nuclear fallout.
It is not possible to completely avoid background radiation because it is naturally occurring and surrounds us constantly. Exposure levels can vary by location, but it cannot be entirely escaped.
MULTIPLE CHOICE TYPE
1. A radioactive substance emits radiations:
(a) α, β and γ simultaneously
(b) in the order α, β and γ one by one
(c) X-rays and γ-rays
(d) α or β.
Ans: (d) α or β.
2. In β-emission from a radioactive substance, an electron is ejected. This electron comes from:
(a) the outermost orbit of atom
(b) the inner orbits of atom
(c) the surface of substance
(d) the nucleus of atom.
Ans: (d) the nucleus of atom.
3. The least penetrating radiation is:
(a) α-particles
(b) β-particles
(c) X-rays
(d) γ radiations.
Ans: (a) α-particles
4. The radiation suffering the maximum deflection in a magnetic field is:
(a) α-particles
(b) β-particles
(c) X-rays
(d) γ radiations.
Ans: (b) β-particles
EXERCISE 12(B)
1. What do you mean by nuclear energy? What is responsible for its release?
Ans: The release of this energy is primarily responsible for two processes:
Nuclear Fission: The splitting of a heavy, unstable nucleus (like Uranium-235 or Plutonium-239) into smaller nuclei when bombarded with neutrons.
Nuclear Fusion: The combining of two light nuclei (like Hydrogen isotopes) to form a heavier nucleus, which occurs under extreme heat and pressure, such as in the sun.
The energy is released due to the conversion of a small amount of mass into a large amount of energy, as described by Einstein’s equation, E = mc². This conversion happens because the total mass of the resulting particles is less than the mass of the original nucleus, and this “missing mass” (mass defect) is transformed into energy.
2. Write down the Einstein’s mass-energy equivalence relation, explaining the meaning of each symbol used in it.
Ans:
Einstein’s mass-energy equivalence relation is:
E=mc *2
Meaning of each symbol:
E → Energy (in joules, J)
m → Mass (in kilograms, kg)
c → Speed of light in vacuum (3×10*8m/s)
This equation states that mass can be converted into energy, and energy can be converted into mass.
3.(a) What is a.m.u? Express 1 a.m.u. in MeV.
(b) Write the approximate mass of a proton, neutron and electron in a.m.u.
Ans:(a) a.m.u. stands for atomic mass unit. It is a standard unit of mass used to express the masses of atoms and subatomic particles.
1 a.m.u. = 931 MeV (approximately).
(b) Approximate masses in a.m.u.:
Proton: 1.007276 a.m.u.
Neutron: 1.008665 a.m.u.
Electron: 0.00054858 a.m.u.
4. What is nuclear fission? Name the particle used for it. Write one fission reaction.
Ans: Nuclear fission is the process in which a heavy nucleus (such as uranium or plutonium) splits into two smaller nuclei of nearly equal masses, along with the release of a large amount of energy.
The particle used for nuclear fission is the neutron.
One fission reaction is:
92235U+01n⟶56141Ba+3692Kr+301n+Energy
Here, a uranium-235 nucleus absorbs a neutron and splits into barium-141 and krypton-92, releasing three neutrons and a significant amount of energy in the process.
5.(a) Name two isotopes of uranium.
(b) Which of the isotope mentioned in part (a) above is easily fissionable? Give reason.
(c) State whether the neutron needed for fission reaction of the isotope mentioned in part (b) above, is slow or fast?
Ans: a) Name two isotopes of uranium.
Two well-known isotopes of uranium are Uranium-235 and Uranium-238.
(b)Give reason.
Out of the two, Uranium-235 is the one that is easily fissionable. The reason for this lies in its nucleus; when it captures an extra neutron, it becomes extremely unstable. This instability causes the nucleus to split apart almost instantly into two smaller nuclei, a process which releases a tremendous amount of energy.
(c)For an efficient fission chain reaction with Uranium-235, the neutrons need to be slow-moving, which are also called thermal neutrons. Fast neutrons are much more likely to simply pass through or be captured without causing the nucleus to split, whereas slow neutrons are captured more easily, making the fission process far more effective.
6. Write the approximate value of the energy released in the fission of one nucleus of 92235UWhat is the reason for it?
Ans: Approximate energy released per fission of a ²³⁵U nucleus:
About 200 MeV (or 3.2 × 10⁻¹¹ J).
Reason for this energy release:
The total mass of the fission fragments and neutrons produced is less than the mass of the original ²³⁵U nucleus and the incident neutron. This mass defect (missing mass) is converted into a large amount of energy according to Einstein’s equation,
E=Δmc/2
, where
Δm is the mass defect and
c is the speed of light.
7. Complete the following nuclear fission reactions:
(a) 92235U+01n→??Ba+?92Kr+301n+….
(b) 92235U+01n→?148La+35?Br+….
(c) 92235U+01n→??Ba+3692Kr+301n+energy
(d) 92235U+01n→?148La+3585Br+301n+energy
Ans:
(a) 92235U+01n→??Ba+?92Kr+301n+energy
(b) 92235U+01n→?148La+35?Br+energy
(c) 92235U+01n→??Ba+3692Kr+301n+energy
(d) 92235U+01n→?148La+3585Br+301n+energy
8. What do you mean by the chain reaction in nuclear fission? How is it controlled?
Ans:
A nuclear fission chain reaction is a self-sustaining process where splitting an atomic nucleus releases neutrons, which go on to cause further fission in a continuous sequence.
This reaction is managed inside a nuclear reactor using:
Control Rods:. By moving them in or out of the reactor core, operators control how many neutrons are available to sustain the chain reaction.
Moderator: Substances such as water or graphite slow down the fast neutrons produced by fission. Slower neutrons are more effective at causing additional fission in fuel like uranium-235, helping maintain a steady and controlled reaction.
Through these methods, the chain reaction is kept stable, ensuring safe and consistent energy production without escalating into an explosion.
9. State two uses of nuclear fission.
Ans: Nuclear fission has two primary applications.
The first is generating nuclear power. In this controlled process, fission reactions in reactors produce heat. This heat creates steam that spins turbines, generating electricity for residential and industrial use.
The second is nuclear weapons. These devices use uncontrolled, rapid fission chain reactions to produce a massive, instantaneous explosion for military purposes.
10. Give two differences between the radioactive decay and nuclear fission.
Ans: Radioactive decay and nuclear fission are distinct nuclear processes. Radioactive decay is a spontaneous and natural event where an unstable nucleus randomly releases radiation to achieve stability, and its rate cannot be controlled. In contrast, nuclear fission is typically an induced reaction where a heavy nucleus, after absorbing a neutron, splits apart. This splitting is not spontaneous and can be controlled, as in nuclear reactors. Furthermore, while decay involves a minor transformation of the original nucleus, fission shatters a heavy nucleus into two or more lighter fragments, releasing a vastly greater amount of energy and additional neutrons in the process.
11.(a) What is nuclear fusion? Give one example and write its nuclear reaction.
(b) What other name is given to nuclear fusion? Give reason.
Ans: (a) Nuclear fusion is a process where two or more lighter atomic nuclei combine to form a single, heavier nucleus. This process releases an enormous amount of energy because the mass of the resulting nucleus is slightly less than the sum of the original masses, and this lost mass is converted into energy according to Einstein’s equation,
E=mc 2 .
Example: The fusion of hydrogen isotopes to form helium in the Sun, which is the primary source of solar energy.
Nuclear Reaction:
12H+13H→24He+01n+Energy
(Deuterium + Tritium → Helium + Neutron + Energy)
(b) This is because extremely high temperatures (on the order of millions of degrees Celsius) are required to provide the nuclei with enough kinetic energy to overcome their mutual electrostatic repulsion (the Coulomb barrier) and come close enough for the strong nuclear force to bind them together.
12. Why is a very high temperature required for the process of nuclear fusion? State the approximate temperature required.
Ans: Nuclear fusion requires an extremely high temperature, approximately 15 million degrees Celsius in the core of the Sun and around 100 million degrees Celsius for artificial fusion on Earth, for one fundamental reason: to overcome the powerful electrostatic repulsion between atomic nuclei.
Atomic nuclei are positively charged and naturally repel each other due to the electrostatic force. For fusion to occur, these nuclei must be brought close enough for the attractive strong nuclear force to take over and bind them together. This only happens if the nuclei are moving towards each other at tremendously high speeds. A high temperature is essential because it provides the nuclei with immense kinetic energy, making them move so fast and collide with such violence that they can overcome their mutual repulsion and fuse.
13.(a) Write one nuclear fusion reaction.
(b) State the approximate value of energy released in the reaction mentioned in part (a).
(c) Give reason for the release of energy stated in part (b).
Ans: (a) One example of a nuclear fusion reaction is the fusion of deuterium and tritium nuclei to form a helium nucleus and a neutron:
12H+13H→24He+01n
(b) The energy released in this reaction is approximately 17.6 MeV (million electron volts).
(c) The energy is released due to the mass defect — the total mass of the resulting particles (24He+01 n) is slightly less than the total mass of the initial particles (deuterium + tritium). This lost mass is converted into a large amount of energy, as described by Einstein’s equation E=mc *2 .
14. Complete the following fusion reactions:
(a) 23He+12H→24He+11H+energy
(b) 12H+12H→23He+01n+energy
Ans:
(a) Reaction: ³₂He + ²₁H → ⁴₂He + ¹₁H + energy
This equation describes a nuclear fusion process where a nucleus of Helium-3 and a nucleus of Deuterium (Hydrogen-2) combine. The result of this fusion is the formation of a stable Helium-4 nucleus, along with a leftover proton (which is a Hydrogen-1 nucleus), and a release of energy.
When we check the balance of the reaction, it holds true on both sides. The total mass number (the superscript) is 3 + 2 = 5 on the left, and 4 + 1 = 5 on the right. Similarly, the total atomic number (the subscript) is 2 + 1 = 3 on the left, and 2 + 1 = 3 on the right. This balance confirms that the reaction is correctly represented.
(b) Reaction: ²₁H + ²₁H → ³₂He + ¹₀n + energy
This equation shows another fusion path, one where two Deuterium (²₁H) nuclei collide and fuse together. The products of this particular collision are a Helium-3 nucleus and a neutron (¹₀n), accompanied by a significant release of energy.
Looking at the conservation laws, the total mass number is 2 + 2 = 4 initially, and the products also add up to 3 + 1 = 4. For the atomic number, the two deuterium nuclei contribute 1 + 1 = 2, which matches the Helium-3 nucleus’s atomic number of 2, as the neutron carries no charge (0).
These kinds of reactions are not just theoretical; they are the fundamental processes that power stars like our Sun, where immense pressure and heat force atomic nuclei to merge. Scientists are actively researching how to control and sustain such fusion reactions on Earth, with the goal of creating a nearly limitless and clean source of energy for the future.
15.(a) Name the process, nuclear fission or nuclear fusion, in which the energy released per unit mass is more?
(b) Name the process, fission or fusion which is possible at ordinary temperature.
Ans:
(a) Nuclear fusion
(b)Nuclear fission
16.(a) State one similarity in the process of nuclear fission and fusion.
(b) State two differences between the process of nuclear fission and fusion.
Ans: (a) Similarity between Nuclear Fission and Fusion
Both fission and fusion are nuclear reactions that release a massive amount of energy due to the conversion of a small quantity of mass into energy, as explained by Einstein’s equation
E=mc*2
(b) Differences between Nuclear Fission and Fusion
Process
Fission: A heavy nucleus (e.g., Uranium-235) splits into lighter nuclei.
Fusion: Light nuclei (e.g., Hydrogen isotopes) combine to form a heavier nucleus.
Conditions and Waste
Fission: Occurs at relatively lower temperatures and produces long-lasting radioactive waste.
Fusion: Requires extremely high temperature and pressure, and generates little long-lived radioactive waste.
17. Give two examples of nuclear fusion.
Ans:
Two examples are:
The Sun and Stars: In the core of the Sun, hydrogen nuclei fuse together under extreme heat and pressure to form helium, which produces the sunlight and heat that reaches Earth.
Hydrogen Bomb: A hydrogen bomb is a man-made weapon that uses the energy from a fission explosion to trigger the fusion of hydrogen isotopes, resulting in an immensely powerful explosion.
18. What is the source of energy of sun or stars?
Ans:The source of the sun’s and stars’ energy is a process called nuclear fusion.
Deep inside the sun’s core, the temperature and pressure are incredibly high. Under these extreme conditions, the nuclei of small atoms are forced to collide and stick together. Specifically, the nuclei of the hydrogen atoms fuse to form a larger helium atom.
However, the mass of the resulting helium nucleus is slightly less than the total mass of the four hydrogen nuclei that started the process. This tiny amount of “lost” mass does not just disappear; according to Einstein’s famous equation, E=mc², it is converted into a massive amount of energy. In this equation, ‘E’ is energy, ‘m’ is mass, and ‘c’ is the speed of light—which is an enormous number. This means that even a very small amount of mass converts into a tremendous amount of energy.
This continuous, powerful nuclear fusion reaction in the core is what makes the sun and all other stars shine so brightly, producing immense heat and light for billions of years.
19. Name the following nuclear reactions:
(a) 92235U+01n→3894Sr+54140Xe+301n+γ
(b) 12H+13H→24He+01n+γ
Ans: nuclear fission
nuclear fusion
MULTIPLE CHOICE TYPE
1. The particle used in nuclear fission for bombardment is:
(a) alpha particle
(b) proton
(c) beta particle
(d) neutron
Ans: (d) neutron
2. The temperature required for the process of nuclear fusion is nearly:
(a) 1000 K
(b) 104
Ans: (b) 104