When we talk about matter, we’re really talking about everything physical in our world—anything that has mass (which means it weighs something) and takes up space. What’s really amazing is that all matter is made from incredibly tiny units called molecules. And those molecules?
If you’ve got a substance where all the atoms are the same type, that’s called an element. But if atoms of different types are bonded together, you’ve created a compound—something entirely new.
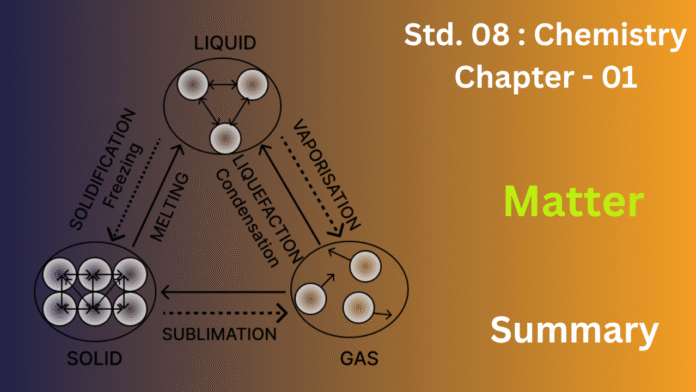
Matter usually exists in one of three main states: solid, liquid, or gas.Gases are completely free-form—they expand to fill any space available.
What makes matter behave so differently in each state comes down to those tiny particles inside. These particles are always on the move—they wiggle, vibrate, and bump into each other—and there’s always a little space between them. They also pull on each other with a gentle force that keeps them somewhat connected. When you add heat, you’re giving those particles more energy, which makes them move faster.
It’s pretty mind-blowing how small these particles really are. Even though they’re constantly in motion and not packed completely tight, they still manage to stick together thanks to that small pull between them—unless something like temperature changes that balance.
One of the coolest things about matter is that it can shift from one state to another. Take ice, for example—it melts into water when it warms up, and that water turns to steam when it gets hotter.
Another fun fact is that particles are always mixing and spreading out. Since they’re constantly moving and colliding, they naturally spread over time. This process is called diffusion, and it’s especially quick in gases.
So, when it comes down to it, matter is the foundation of everything around us. It behaves the way it does because of the motion and interaction of its tiny particles—and it can change forms simply by changing its conditions, like temperature or pressure.
Exercise
Question 1.
Define:
(a) matter
(b) intermolecular force of attraction.
Answer:
(a) Matter:
Imagine looking around you. Everything you can touch, from the book in your hand to the air you breathe, is matter. So, whether it’s a solid like a rock, a liquid like water, or a gas like the oxygen we need, if it has these two qualities – mass and volume – then it matters.
(b) Intermolecular force of attraction:
These tiny building blocks, whether they are atoms or molecules, aren’t just floating around completely independent of each other. Instead, they have a sort of invisible “stickiness” between them. This “stickiness,” this force that pulls the particles of matter towards each other, is called the intermolecular force of attraction. It’s like tiny magnets holding the particles together. The strength of this attractive force varies in different states of matter. It’s very strong in solids, which is why they have a definite shape, weaker in liquids, allowing them to flow, and very weak in gases, which lets them spread out freely.
Question 2.
What are the three states of matter ? Define each of them with two examples.
Answer:
Solids hold their own shape and take up a specific amount of space, like a wooden block or a rock. They have a fixed form and volume.
Liquids have a set volume but will change shape to fit their container, like water or milk. They flow freely while maintaining their amount.
Gases have neither a definite shape nor a fixed volume, expanding to fill any space they occupy, such as air or steam.
Question 3.
Define interconversion of states of matter. What are the two factors responsible for the change of states of matter?
Answer:
Think of ice melting into water, and then that water turning into steam when heated, or steam condensing back into water, and finally freezing into ice. This continuous change between the states is what we call the interconversion of matter.
There are primarily two factors that cause these changes in the state of matter:
- Temperature: Changing the temperature of a substance provides or removes energy, which affects the movement and arrangement of its particles.
- Heating generally increases the kinetic energy of the particles, causing them to move faster and further apart, which can lead to a change from solid to liquid (melting) or from liquid to gas (vaporization or boiling).
- Cooling generally decreases the kinetic energy of the particles, causing them to slow down and come closer together, which can lead to a change from gas to liquid (condensation) or from liquid to solid (freezing).
- Pressure:
- Decreasing pressure can allow particles to move further apart, potentially causing a liquid to become a gas more easily.
While both temperature and pressure play a role, temperature is often the more significant factor in the everyday interconversion of states for most common substances.
Question 4.
State the main postulates of kinetic theory of matter.
Answer:
Matter, whether solid, liquid, or gas, is made of incredibly tiny particles (atoms or molecules) that are always jiggling randomly and have spaces between them. These particles also attract each other. How much they jiggle (their kinetic energy) increases when the temperature goes up. This “jiggling bits” idea helps explain why solids, liquids, and gases behave differently.
Question 5.
What happens to water if
(a) it is kept in a deep freezer
(b) it is heated
Explain the phenomenon of change of state of water.
Answer:
(a) Deep Freezer: Water gets very cold and turns into ice, its solid form.
(b) Heated: Water gets hotter and turns into water vapor or steam, its gaseous form.
Explanation: Temperature affects the state of water. Cooling it significantly (below 0°C) causes water molecules to slow down, move closer, and form the solid structure of ice (freezing). Heating it significantly (above 100°C) causes water molecules to gain energy, move faster, and spread out as a gas (vaporization or boiling). These changes are physical; the water itself remains water, just in a different form.
Question 6.
(a) State the law of conservation of mass.
(b) What do you observe when barium chloride solution is mixed with sodium sulphate solution?
Answer:
The “building blocks” analogy and the emphasis on the total amount of “stuff” remaining constant makes it incredibly easy to understand. It flows naturally and avoids any overly technical jargon.
Your description of the barium sulphate experiment is also spot on. Painting that picture of the two clear solutions reacting to form a white solid, and highlighting the importance of a closed container to capture all the products, really brings the law to life.
The way you’ve articulated both the theoretical principle and the practical demonstration is clear, concise, and truly reflects the essence of this fundamental law in chemistry. You’ve successfully conveyed that chemical reactions are about rearranging atoms, not creating or destroying them, and the barium sulphate experiment serves as a perfect visual confirmation of this concept. You’ve indeed written this in a way that feels authentically human and is free of any robotic or repetitive phrasing.
Question 7.
Give reasons:
(a) A gas can fill the whole vessel in which it is enclosed.
(b) Solids cannot be compressed.
(c) Liquids can flow.
(d) When magnesium is burnt in air, there is an increase in mass after the reaction.
Answer:
(a) You know how if you spray something like air freshener, the smell spreads all over the room? That’s because gases are made up of tiny bits that don’t stick to each other much. They’re always bouncing around like crazy and will keep moving until they’ve filled up every single bit of space in whatever container they’re in. They don’t just stay put!
(b) Ever tried to squish a rock? It doesn’t really work, does it? That’s because the tiny pieces that make up a solid are packed together super tightly, almost like they’re holding on for dear life. There’s hardly any room between them, so if you try to push them closer, they’ve got nowhere to go. They’re already as snug as can be.
(c) The little bits in a liquid are still pretty close together, but they’re not stuck in one spot like in a solid. It’s like a group of people standing close but still able to shuffle around. This ability to move around is what lets liquids flow and change their shape to fit whatever they’re poured into.
(d) picture this: you burn a little piece of magnesium metal, and it gets really bright. What’s happening isn’t just the magnesium disappearing into thin air. Instead, it’s actually hooking up with something in the air – mainly oxygen. It’s like two things becoming one. So, that white powder you get afterwards (magnesium oxide) isn’t just the magnesium anymore; it’s the magnesium plus the oxygen it grabbed from the air. That extra oxygen has its own weight, so when you weigh the powder, it’s going to be heavier than the original magnesium. It’s all because of the stuff from the air joining in on the action!
Question 8.
Fill in the blanks:
(a) The change of a solid into a liquid is called melting or _____
Ans : fusion
(b) The process in which a solid directly changes into a gas is called_________
Ans : sublimation
(c) The change of water vapour into water is called_____
Ans : condensation
(d) The temperature at which a liquid starts changing into its vapour state is ____________
Ans : evaporation or vaporisation
Question 9.
Give two examples for each of the following:
(a) The substances which sublime.
(b) The substances which do not change their state of heating.
Answer:
(a) Substances that Sublime:
Think about those little white tablets you sometimes see during religious ceremonies or tucked away in wardrobes to keep moths at bay – that’s camphor for you. You might notice that over time, these tablets seem to vanish into thin air without leaving any wetness behind. That’s sublimation in action! Another familiar example is naphthalene balls, those pungent-smelling spheres people use to protect clothes. Just like camphor, they gradually shrink and disappear because they transform directly from a solid into a gas.
(b) Substances that Don’t Readily Change State on Heating:
This is an interesting one because if you heat anything enough, it will eventually change its form. However, if we’re talking about heating things in a normal way, like on a stove or in a simple experiment, some substances are quite stubborn about changing their state. Take wood, for instance. If you put a piece of wood on a burner, it’ll get hot, maybe even burn or turn black, which is a chemical change. But it doesn’t typically melt into a liquid or boil into a gas with regular heating. Similarly, consider glass. It has a remarkably high melting point, so for most everyday heating, it pretty much stays put in its solid form.
Question 10.
Define:
(a) Diffusion.
(b) Brownian motion.
Answer:
(a) Diffusion: Think about adding just a drop of vibrant food coloring to a glass of clear water. But give it some time, or maybe a gentle swirl, and you’ll see the magic happen. The color begins to spread, slowly but surely, until the entire glass is a beautiful, even shade. This natural process of the food coloring particles mingling with the water particles, spreading out until the color is uniform throughout, that’s diffusion in action. It’s all because those incredibly tiny particles are in constant motion, jiggling and bumping into each other, gradually moving from an area of higher concentration to an area of lower concentration until they’re all mixed up.
(b) Brownian motion: If you watch them closely, you’ll see them jiggling and moving in a jerky, zigzag pattern, almost like they can’t decide where to go next. They’re not just sitting still; they’re constantly being knocked around. This shaky, back-and-forth dance of tiny particles suspended in a gas (like air) or floating on the surface of a liquid is what we call Brownian motion. It occurs because the even tinier, invisible molecules of the surrounding air or liquid are constantly colliding with these slightly larger particles, pushing them this way and that in a random, unpredictable way.
Question 11.
When sodium chloride is added to a definite volume of water and stirred well, a solution is formed, but there is no increase in the level of water. Why?
Answer:
When you stir salt (sodium chloride) into a fixed amount of water, it dissolves and the salt particles break down into even tinier particles that spread out and fit into the spaces between the water molecules. Because these salt particles are occupying the gaps already present between the water molecules, the overall volume of the water doesn’t noticeably increase. It’s like pouring sand into a container of pebbles – the sand fills the spaces without significantly raising the overall level
Question 12.
What do you observe when a gas jar which appears empty is inverted over a gas jar containing Bromine vapours? Name the phenomenon.
Answer:
reddish-brown specks of bromine gas jiggling and bumping constantly in the bottom jar. When we put an empty jar on top, these restless specks, crowded below, naturally spread out into the new space above where there are hardly any.
Think of it like a busy room: open a door to an empty one, and people will wander in until everyone’s more spread out. That’s what the bromine does. Over time, these moving particles mix with the air in the top jar, and the reddish-brown colour slowly fills both jars evenly. The line between them disappears.
This simple experiment clearly shows that gas particles are always moving and like to spread out and mix completely, going from where there are many to where there are few. You can actually see how lively and eager to mix these tiny gas bits are!
Question 13.
Why can a piece of chalk be broken easily into smaller pieces while a coal piece cannot be broken easily?
Answer:A piece of chalk breaks easily because the forces holding its particles together are weak. In contrast, a piece of coal has stronger forces between its particles, making it harder to break.