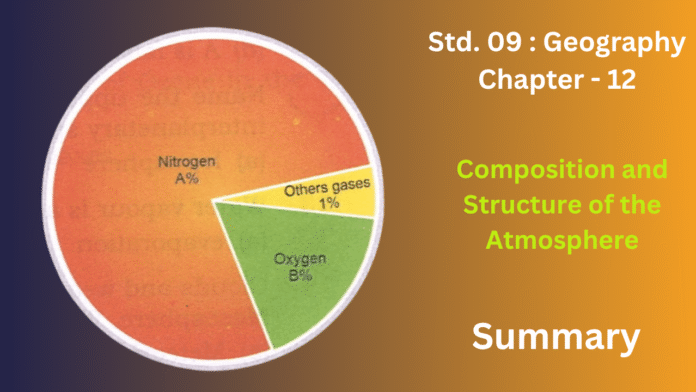
The Earth’s atmosphere is a vital gaseous shield, held in place by gravity, that makes life on our planet possible. It’s primarily composed of Nitrogen, making up about 78%, which helps to temper the reactivity of oxygen and is indirectly crucial for biological cycles. Oxygen, at roughly 21%, is fundamental for both respiration and combustion. Though a small percentage, Carbon Dioxide (0.03% to 0.04%) plays a critical dual role: it’s essential for plant photosynthesis and acts as a greenhouse gas, trapping heat to maintain Earth’s habitable climate. Other minor gases, like argon, neon, and helium, along with variable amounts of water vapor for the hydrological cycle and dust particles that aid cloud formation, complete this complex mixture.
The Troposphere, the lowest and densest layer extending to an average of 13 km, is where all our weather occurs, with temperatures decreasing as altitude increases. Above this is the Stratosphere, reaching up to 50 km, known for its stable conditions and, most importantly, the Ozone Layer. This critical shield, concentrated between 20-30 km, absorbs harmful ultraviolet radiation from the sun, leading to a temperature increase within this layer. Next is the Mesosphere, extending to about 80-85 km, which is the coldest part of the atmosphere and where most meteors burn up.
This layer includes the Ionosphere, a region of charged particles vital for reflecting radio waves and creating the stunning auroras. The outermost layer is the Exosphere, where the atmosphere gradually fades into outer space, containing extremely sparse light gases like hydrogen and helium that can escape Earth’s gravity. Together, these atmospheric layers and their diverse components are indispensable, supplying essential gases, regulating global temperatures, and providing crucial protection from solar radiation and incoming space debris, all of which are fundamental for sustaining life.
Exercises
I. Short Answer Questions.
Question 1.
What is known as the atmosphere ?
Ans:
The atmosphere refers to the vital layer of gases enveloping a celestial body, such as Earth, maintained by the force of gravity. On our planet, this gaseous blanket is commonly known as “the air.”
- Gas Mixture:The small remaining fraction includes argon, carbon dioxide, and minute quantities of other noble gases like neon and helium, alongside methane and hydrogen. Additionally, it contains fluctuating amounts of water vapor and tiny particulate matter like dust.
- Protective Barrier: It serves as a natural shield, guarding life on Earth from detrimental solar radiation, specifically ultraviolet rays which are absorbed by the ozone layer within the atmosphere. It also defends against incoming meteoroids, most of which disintegrate upon atmospheric entry due to frictional heating.
- Thermal Moderator: The atmosphere plays a crucial role in moderating Earth’s temperature, preventing severe temperature swings between day and night. Gases such as carbon dioxide and water vapor act as natural greenhouse agents, trapping some of the sun’s warmth and ensuring a hospitable temperature for life.
- Sustainer of Life: It delivers the essential gases necessary for the sustenance of life forms; oxygen is vital for animal respiration, while carbon dioxide is indispensable for plant photosynthesis.
- Driver of Weather and Climate: All atmospheric events, from cloud formation and precipitation to severe storms, originate within this gaseous layer, particularly in its lowest stratum. Furthermore, it forms the bedrock of Earth’s climatic system.
Question 2.
State the gaseous composition of the atmosphere.
Ans:
The Earth’s atmospheric blanket is primarily a mixture of several key gases, each playing a vital role.While not directly breathable by most life forms in its atmospheric state, it’s crucial for diluting oxygen and is fixed into usable forms by certain organisms. Following closely is Oxygen (O₂), comprising about 21%, which is absolutely essential for the respiration of living beings and for combustion processes.
The most significant among these is Argon (Ar), an inert gas making up around 0.93%. **Carbon Dioxide (CO₂) **, though present in a much smaller concentration (roughly 0.03% to 0.04%), is disproportionately important. It’s a cornerstone for photosynthesis in plants and acts as a significant greenhouse gas, trapping heat and contributing to the Earth’s moderate temperatures. Trace amounts of other noble gases like Neon (Ne), Helium (He), Krypton (Kr), and Xenon (Xe) are also present. Furthermore, variable components like water vapor (H₂O), crucial for the hydrological cycle and temperature regulation, and dust particles, which serve as nuclei for cloud formation, are also integral parts of the atmosphere’s overall composition.
Question 3.
Mention any three functions of the atmosphere.
Ans:
Here are three key functions of the atmosphere:
- Sustaining Life: The atmosphere provides the essential gases necessary for all known life forms on Earth. It contains oxygen, critical for the respiration of animals and many microorganisms, and carbon dioxide, which is vital for plants to perform photosynthesis – the process that forms the base of most food chains. Without these specific gases in their current proportions, complex life as we know it could not exist.
- Regulating Temperature: The atmosphere acts like a thermal blanket for the planet, preventing extreme temperature swings. It traps some of the sun’s heat through the greenhouse effect, keeping Earth warm enough to support liquid water and life. At the same time, it helps to distribute heat around the globe through winds and weather patterns, moderating temperatures between day and night and across different latitudes, thus creating a more stable and habitable environment.
- Protecting from Harm: The atmosphere serves as a crucial protective shield against various external threats. It absorbs and scatters harmful ultraviolet (UV) radiation from the sun, particularly through the ozone layer, safeguarding living organisms from DNA damage and other adverse health effects. Additionally, it acts as a defense against incoming space debris like meteoroids; most of these burn up due to friction upon entering the dense layers of the atmosphere, preventing them from impacting the Earth’s surface.
Question 4.
Name the four layers of the atmosphere.
Ans:
- Troposphere: It’s characterized by a natural cooling trend as altitude increases, due to decreasing density and distance from the warm ground.
- Stratosphere: Resting above the troposphere, this layer exhibits a fascinating temperature inversion, meaning temperatures increase with height. This warming is directly attributed to the vital ozone layer within it, which efficiently absorbs damaging ultraviolet radiation from the sun, converting it into heat.
- Mesosphere: Positioned above the stratosphere, this layer marks a return to falling temperatures with increasing altitude, making it the coldest region within our atmosphere. It acts as a protective shield, as most incoming meteors disintegrate here due to atmospheric friction.
- Thermosphere: The outermost significant layer, the thermosphere experiences a dramatic rise in temperature with increasing height. This extreme heating is a result of the sparse gas molecules directly absorbing high-energy solar radiation. This layer also contains the ionosphere, a region of charged particles essential for bouncing radio waves and the site of the mesmerizing auroral displays.
Question 5.
What is known as the troposphere ?
Ans:
The troposphere represents the Earth’s atmosphere at its most dynamic, serving as the layer directly above our planet’s surface. It’s the atmospheric zone critical to human existence and the birthplace of virtually all meteorological events.
Delving into its attributes:
- Proximity to Earth: This layer stretches from ground level to an average altitude of approximately 13 kilometers (8 miles). Its vertical extent isn’t uniform; it’s shallower near the poles (roughly 8 km or 5 miles) and expands to about 18 km (11 miles) at the equator.
- Temperature Decline with Ascent: A hallmark of the troposphere is the consistent drop in temperature as one ascends. This phenomenon, known as the environmental lapse rate, typically sees a decrease of about 6.5°C for every kilometer climbed (or 3.5°F per 1,000 feet).
- Origin of Weather: Given its abundance of water vapor, marked temperature differences, and continuous air circulation, the troposphere functions as the “weather engine” of our planet. All forms of atmospheric phenomena—including clouds, rainfall, snowfall, lightning storms, and winds—originate and unfold within this layer.
- The Tropopause: At this transitional point, the descent in temperature with altitude ceases, and temperatures either stabilize or begin a slight ascent, signaling the start of the subsequent atmospheric layer, the stratosphere.
Question 6.
Mention the chief characteristics of the stratosphere.
Ans:
The stratosphere is the second major layer of Earth’s atmosphere, situated directly above the troposphere and extending upwards to about 50 kilometers (31 miles) above the Earth’s surface. It possesses several distinct characteristics:
First and foremost, a defining feature of the stratosphere is its temperature inversion. This warming trend is primarily due to the presence of the Ozone Layer. Located predominantly between 20 to 30 kilometers (12 to 19 miles) within the stratosphere, this vital layer of ozone gas (O₃) absorbs most of the sun’s harmful ultraviolet (UV) radiation. This absorption converts UV energy into heat, causing the stratospheric temperature to rise with height.
Secondly, the stratosphere is characterized by its stability and calmness. Due to the temperature inversion, there is very little vertical air movement, unlike the turbulent troposphere where weather phenomena occur. This stability makes the stratosphere an ideal region for long-haul commercial aircraft to fly, as it minimizes turbulence and provides smoother flight conditions. Consequently, clouds and other weather events are virtually absent in this layer.
By absorbing most of the biologically damaging UV radiation, the ozone layer prevents it from reaching the Earth’s surface, thus safeguarding living organisms from skin cancer, cataracts, and damage to ecosystems. The stratopause marks the upper boundary of the stratosphere, where temperatures reach their peak for this layer before beginning to decrease in the mesosphere above.
Question 7.
In which layer of atmosphere do all weather conditions occur ?
Ans:
All weather conditions occur in the Troposphere.
This is the lowest and densest layer of Earth’s atmosphere, extending from the surface up to an average of about 13 kilometers (though its height varies, being thinner at the poles and thicker at the equator). It’s where we experience phenomena like clouds, rain, snow, wind, and storms, as well as the general daily temperature changes.
Question 8.
Name the constituent gases of the atmosphere which scientists consider responsible for climate change.
Ans:
Scientists widely identify the following constituent gases in the atmosphere as primarily responsible for climate change due to their heat-trapping properties:
- Carbon Dioxide (CO₂): The most significant contributor, largely from burning fossil fuels, deforestation, and industrial processes.
- Methane (CH₄): A potent gas emitted from agriculture (livestock, rice cultivation), fossil fuel production, and waste decomposition.
- Nitrous Oxide (N₂O): Primarily released from agricultural activities (fertilizer use), fossil fuel combustion, and industrial processes.
- Fluorinated Gases (F-gases): A group of synthetic industrial gases including hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF₆), used in refrigerants, aerosols, and various industrial applications. Though present in smaller concentrations, they have a very high global warming potential.
Question 9.
What is known as the ozone layer ?
Ans:
Its essential role is to absorb most of the Sun’s harmful ultraviolet (UV) radiation, especially the dangerous UV-B and UV-C types. Without this protective filter, increased UV exposure would severely harm human health (e.g., skin cancer, cataracts), damage ecosystems, and degrade materials. This crucial shield is naturally maintained by a continuous cycle of ozone formation and breakdown, driven by solar UV radiation, acting as an indispensable safeguard for life on Earth.
Question 10.
What is leading to depletion of the ozone layer in the atmosphere ?
Ans:
The primary cause of ozone layer depletion is the emission of certain human-made chemicals into the atmosphere, collectively known as Ozone-Depleting Substances (ODS).
These substances, such as:
- Chlorofluorocarbons (CFCs)
- Hydrochlorofluorocarbons (HCFCs)
- Halons
- Carbon tetrachloride
- Methyl bromide
were historically used in refrigerants, aerosol propellants, fire extinguishers, solvents, and foam-blowing agents. Once released, these highly stable chemicals slowly rise into the stratosphere. These atoms then act as catalysts, repeatedly destroying ozone molecules much faster than ozone can naturally regenerate, leading to a thinning of the protective ozone layer.
Question 11.
What is known as Global Warming?
Ans:
Global warming refers to the ongoing, long-term increase in Earth’s average surface temperature. This phenomenon is primarily driven by human activities that release large amounts of greenhouse gases into the atmosphere, intensifying the natural greenhouse effect.
Here’s a breakdown of the concept:
- The Greenhouse Effect: Naturally, certain gases in the atmosphere, such as carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O), trap some of the heat radiating from Earth’s surface, preventing it from escaping into space.
- Human Impact: Since the Industrial Revolution, human activities, particularly the burning of fossil fuels (coal, oil, and natural gas) for energy, deforestation, and certain agricultural practices, have significantly increased the concentration of these greenhouse gases in the atmosphere.
- Enhanced Trapping of Heat: With higher concentrations of greenhouse gases, more heat gets trapped, leading to a rise in the planet’s overall temperature. This amplified warming is what we call global warming.
- Consequences: The consequences of global warming are far-reaching and include rising sea levels (due to melting glaciers and ice sheets, and thermal expansion of water), more frequent and intense heatwaves, altered precipitation patterns leading to more severe droughts or floods, increased intensity of storms, and disruptions to ecosystems and biodiversity.
Question 12.
Name important Greenhouse gases.
Ans:
Here are the important greenhouse gases: The most important greenhouse gases, which trap heat in Earth’s atmosphere and contribute to the greenhouse effect, are:
- Carbon Dioxide (CO₂): This is the primary greenhouse gas emitted by human activities, mainly from burning fossil fuels (coal, oil, and natural gas), deforestation, and industrial processes like cement production. While its global warming potential per unit is lower than some other gases, its sheer volume of emissions makes it the most significant contributor to global warming.
- Methane (CH₄): A potent greenhouse gas, methane is released from sources such as livestock farming, landfills, natural gas and oil systems, and rice cultivation. It has a much higher heat-trapping ability than CO₂ over a shorter period, though it doesn’t remain in the atmosphere as long as CO₂.
- Nitrous Oxide (N₂O): Emitted from agricultural activities (especially the use of nitrogen-based fertilizers), fossil fuel combustion, and industrial processes, nitrous oxide is a powerful greenhouse gas with a long atmospheric lifetime.
- Fluorinated Gases: This category includes a group of synthetic gases like Hydrofluorocarbons (HFCs), Perfluorocarbons (PFCs), Sulfur Hexafluoride (SF₆), and Nitrogen Trifluoride (NF₃). These gases are primarily produced for industrial purposes (e.g., refrigerants, aerosols, fire suppressants) and, despite being present in smaller quantities, have extremely high global warming potentials.
- Water Vapor (H₂O): While often overlooked because it’s naturally occurring and highly variable, water vapor is the most abundant greenhouse gas in the atmosphere. It acts as a feedback mechanism; as temperatures rise due to other greenhouse gases, more water evaporates, leading to more water vapor in the atmosphere, which in turn traps more heat, further accelerating warming.
Question 13.
Name two chief characteristics of the atmosphere.
Ans:
The two chief characteristics of the atmosphere are its composition and its layered structure.
- Composition: The atmosphere is a specific mixture of various gases, with nitrogen (approximately 78%) and oxygen (around 21%) being the most abundant. It also contains smaller but crucial amounts of carbon dioxide, argon, water vapor, and trace gases, as well as solid and liquid particles like dust and aerosols. This unique blend of elements is fundamental because it provides the gases necessary for life (oxygen for respiration, carbon dioxide for photosynthesis) and plays a key role in regulating Earth’s temperature and weather patterns.
- Layered Structure: The atmosphere is not uniform but is divided into distinct layers (troposphere, stratosphere, mesosphere, thermosphere, exosphere) based primarily on temperature variations with altitude. Each layer possesses unique properties and functions. For instance, the troposphere is where all weather occurs, the stratosphere contains the protective ozone layer, and the thermosphere is home to the ionosphere, which is vital for radio communication. This stratification means that different atmospheric processes occur at different heights, contributing to the overall complexity and functionality of the Earth’s gaseous envelope.
Question 14.
What keeps the atmosphere in a dynamic state ?
Ans:
The Earth’s atmosphere is in constant motion, driven by a combination of factors. Solar energy is the primary engine; its uneven distribution across the globe creates temperature differences, prompting warm air to rise and cold air to sink, which in turn generates large-scale atmospheric circulation and global wind patterns. The Earth’s rotation introduces the Coriolis effect, deflecting these winds and influencing the direction of major weather systems. The water cycle, through processes like evaporation and condensation, adds and releases heat, fueling atmospheric instability and contributing to air movement. Additionally, natural pressure differences cause air to flow from high to low pressure zones, resulting in winds of varying strengths. Finally, topography and surface features like mountains and oceans create friction and localized heating or cooling, further influencing atmospheric flow and generating regional wind patterns.
Question 15.
What is called weather ?
Ans:
Weather describes the immediate, localized conditions of the atmosphere at a specific moment and place. It includes variables such as temperature, the amount of moisture in the air (humidity), any form of water falling from the sky (like rain or snow), the velocity and path of air movement (wind), how much of the sky is covered by clouds, and the force exerted by the air (atmospheric pressure). In contrast to climate, which characterizes atmospheric behavior over extended periods, weather is inherently dynamic, frequently shifting within brief intervals, sometimes even within minutes.
II. Explain the following terms/processes in detail
- Global Warming
- Greenhouse Effect
- Normal Lapse Rate of Temperature
- CFCs
Ans:
Global Warming
Global warming refers to the sustained rise in Earth’s mean surface temperature observed since the mid-19th century. This process intensifies the Earth’s natural thermal insulation, a phenomenon known as the greenhouse effect. The observable consequences are diverse and impactful, encompassing a global increase in sea levels, a surge in the frequency and severity of extreme weather events, and widespread disruptions to ecological systems across the globe. Ultimately, global warming represents a critical component of the broader, multifaceted issue of climate change.
Greenhouse Effect
The greenhouse effect is an indispensable, naturally occurring atmospheric process vital for maintaining the Earth’s temperature within a range conducive to life. It operates as follows: solar energy warms the Earth’s surface, which subsequently re-emits this absorbed heat outwards as infrared radiation. Specific atmospheric constituents, known as greenhouse gases (such as carbon dioxide and methane), intercept and absorb a portion of this outgoing thermal energy. This absorption prevents the heat from escaping into outer space, instead causing it to be re-radiated in various directions, including back towards the Earth’s surface, thereby warming the planet. The term “enhanced greenhouse effect” specifically denotes the additional planetary warming that results from elevated concentrations of these heat-trapping gases, primarily due to human industrial and agricultural activities.
Normal Lapse Rate of Temperature
This meteorological phenomenon primarily occurs because the troposphere receives its principal thermal energy from the Earth’s surface. Additionally, as parcels of air ascend, they encounter progressively lower atmospheric pressure, leading them to expand and consequently cool. Furthermore, higher altitudes generally exhibit a reduced density of heat-absorbing gases, which also contributes to the observed temperature decline.
CFCs (Chlorofluorocarbons)
CFCs, or Chlorofluorocarbons, are synthetic chemical compounds composed of carbon, chlorine, and fluorine atoms. Their remarkable persistence allows these compounds to migrate upwards into the stratosphere, where exposure to intense ultraviolet (UV) radiation from the sun triggers their breakdown, releasing chlorine atoms. These liberated chlorine atoms then function as extraordinarily efficient catalysts, cyclically destroying molecules within the crucial stratospheric ozone layer – Earth’s primary protective barrier against detrimental UV radiation. The consequence is that a greater amount of damaging UV radiation penetrates to the planet’s surface. Moreover, CFCs are potent greenhouse gases, significantly contributing to global warming.
III. Long Answer Questions
Question 1.
Describe the structure of the atmosphere.
Ans:
The Earth’s atmosphere, a crucial gaseous envelope sustained by gravity, acts as an indispensable protective barrier for all life on our planet. Its distinct stratification is primarily determined by variations in temperature with increasing altitude, leading to several well-defined layers.
Atmospheric Layers
- Troposphere: This is the lowest atmospheric layer, extending from the Earth’s surface up to approximately 18 kilometers. It is the realm where all life resides and virtually all weather phenomena unfold. Within the troposphere, temperature typically declines with increasing elevation, a pattern known as the normal lapse rate. This layer holds the vast majority of the atmosphere’s total mass and almost all of its water vapor.
- Mesosphere: Here, the temperature once again decreases with ascending altitude, reaching some of the coldest temperatures found within the atmosphere. The mesosphere is notably where most incoming meteors ignite and disintegrate due to friction.
- Thermosphere: Extending from about 85 kilometers up to between 500 and 1,000 kilometers, the thermosphere experiences a dramatic increase in temperature due to the absorption of high-energy solar radiation. Despite these extremely high temperatures, the air in this layer is exceedingly thin, meaning it would feel cold to a human. Contained within the thermosphere is the ionosphere, an ionized region vital for long-distance radio communication and the mesmerizing displays of the auroras (Northern and Southern Lights). Satellites and the International Space Station (ISS) typically orbit within this layer.
- Exosphere: As the outermost atmospheric layer, the exosphere gradually transitions into the vacuum of outer space, beginning at altitudes ranging from 500 to 1,000 kilometers. The air here is extraordinarily tenuous, primarily composed of lightweight gases such as hydrogen and helium, which can eventually escape into space.
Compositional Layers
- Homosphere: This refers to the lower part of the atmosphere, encompassing the troposphere, stratosphere, and mesosphere, extending up to approximately 80 to 100 kilometers. Within the homosphere, atmospheric gases are uniformly mixed due to constant turbulence and circulation, maintaining a relatively consistent composition of about 78% nitrogen and 21% oxygen.
- Heterosphere: Situated above the homosphere, this upper atmospheric region includes most of the thermosphere and the exosphere, starting at around 80 to 100 kilometers.
Question 2.
How does the atmosphere govern life on earth ?
Ans:
The Earth’s atmosphere is a thin, dynamic blanket of gases vital for life. It provides essential gases like oxygen for breathing, carbon dioxide for plants to create food (photosynthesis), and nitrogen for building biological molecules.
Crucially, the atmosphere regulates Earth’s temperature through the natural greenhouse effect, trapping some of the Sun’s heat to maintain a habitable climate. Without it, temperatures would swing wildly, making life impossible.
It also acts as a protective shield, with the ozone layer absorbing harmful UV radiation, and higher layers blocking X-rays, gamma rays, and dangerous cosmic particles. The atmosphere protects us from space debris by burning up most incoming meteoroids before they hit the ground.
Furthermore, it facilitates the water cycle, enabling evaporation, cloud formation, and precipitation, which distributes freshwater globally and drives weather patterns. Finally, the atmosphere maintains atmospheric pressure necessary for life’s physical integrity and the presence of liquid water, and allows sound to travel, making our world audible.
Question 3.
Explain the factors responsible for depletion of ozone in the atmosphere.
Ans:
The primary factors responsible for ozone depletion in the atmosphere are human-made chemicals, specifically Ozone-Depleting Substances (ODS). These substances release highly reactive chlorine and bromine atoms into the stratosphere, where they catalytically destroy ozone molecules.
Key ODS include:
- Chlorofluorocarbons (CFCs): Once widely used in refrigerants, aerosols, and foam blowing agents.
- Halons: Primarily used in fire extinguishers.
- Hydrochlorofluorocarbons (HCFCs): Developed as temporary substitutes for CFCs, but still have ozone-depleting potential.
- Carbon Tetrachloride: An industrial solvent.
- Methyl Chloroform: Used as a cleaning solvent.
- Methyl Bromide: A pesticide used for fumigation.
When these stable chemicals reach the stratosphere, intense ultraviolet (UV) radiation breaks them down, releasing chlorine and bromine atoms.
While some natural phenomena like volcanic eruptions can have a minor, temporary impact, the overwhelming cause of the observed ozone depletion, especially the “ozone hole” over Antarctica, is the accumulation of these long-lived, human-produced chemicals.
Question 4.
Give a description of the recent studies about Global Warming.
Ans:
Recent climate studies reveal an alarming acceleration of global warming, with 2024 setting a new record as the warmest year and the last decade being the hottest on record. The Earth’s temperature has been rising three times faster since 1975 compared to the long-term average. We are rapidly approaching, and in some individual years, temporarily breaching, the 1.5∘C warming limit set by the Paris Agreement, with projections suggesting this threshold could become inevitable by 2028.
This warming trend is intensifying extreme weather events. Stronger storms, more frequent and intense heatwaves and droughts, and a dramatic increase in wildfires (turning some forests into carbon sources) are becoming the new norm globally.
Cryospheric changes show nuanced but concerning trends. While the Arctic continues its significant ice loss, a temporary increase in Antarctic ice mass from dense snowfall in 2021-2023 isn’t reversing the long-term melting trend, particularly at the margins.
Greenhouse gas emissions continue to rise, reaching their highest recorded level in 2023, primarily driven by fossil CO2 from China and India. Worryingly, the world’s forest carbon sink is shrinking due to fires and deforestation, making forests less effective at absorbing carbon. Oceans are absorbing increasing amounts of heat and CO2, leading to warming and acidification, threatening marine ecosystems like Hawaiian reefs.
Advances in climate science are improving modeling accessibility for policy-making, especially for developing nations. Research suggests the cooling effect of aerosols might have been underestimated, implying higher climate sensitivity, and their regulation could contribute to recent warming. While some countries show progress in climate policy, significant implementation gaps remain. Furthermore, emerging research is highlighting the profound psychological impact of climate change on vulnerable communities.
Question 5.
Why should we protect the atmosphere ?
Ans:
Sustaining Life and Regulating Climate
The atmosphere also acts as Earth’s natural blanket, trapping some of the sun’s heat through the greenhouse effect to maintain a habitable temperature (around 15∘C). Without it, our planet would experience extreme temperature swings, making life impossible.
Protection from Harmful Elements
Our atmosphere shields us from dangerous cosmic threats. Denser atmospheric layers block high-energy cosmic rays and X-rays. Additionally, it burns up most meteoroids and space debris before they can impact Earth, protecting the surface from widespread damage.
Supporting Essential Natural Processes
Beyond protection, the atmosphere is integral to the water cycle, regulating precipitation, evaporation, and cloud formation, which distribute fresh water globally. It also drives weather patterns like wind and rain, shaping our diverse ecosystems. Furthermore, the atmosphere is the medium through which sound waves travel, enabling communication and the sounds of our world.
Consequences of Neglect
Failing to protect our atmosphere, primarily due to human activities like burning fossil fuels, leads to severe consequences:
- Climate change and global warming: Resulting in rising temperatures, extreme weather, and sea-level rise.
- Air pollution: Causing respiratory issues and acid rain.
- Ozone depletion: Increasing exposure to harmful UV radiation.
- Loss of biodiversity: As habitats are destroyed.
- Disruption of the water cycle: Leading to more frequent droughts or floods.
Question 6.
What is known as the Antarctic ozone hole ?
Ans:
The “Antarctic ozone hole” refers to a significant and recurring depletion of the stratospheric ozone layer over the Antarctic continent, observed annually during the Southern Hemisphere’s spring (roughly August to October).
This severe depletion is primarily caused by human-made chemicals, especially chlorofluorocarbons (CFCs) and halons, combined with unique meteorological conditions present over Antarctica during its extremely cold winter:
- Polar Stratospheric Clouds (PSCs): The extremely low temperatures in the Antarctic stratosphere allow these special clouds to form.
- Chemical Reactions: These PSCs provide surfaces for chemical reactions that convert inactive forms of chlorine and bromine (from CFCs/halons) into highly reactive forms.
- Polar Vortex: A strong, circling wind pattern (the polar vortex) isolates the cold air over Antarctica, preventing it from mixing with warmer, ozone-rich air from other regions.
- Sunlight: When sunlight returns in spring, it triggers these activated chlorine and bromine compounds to rapidly destroy ozone molecules in a catalytic cycle, meaning a single chlorine atom can destroy thousands of ozone molecules.
The result is a substantial reduction in ozone levels, allowing more harmful ultraviolet (UV) radiation from the sun to reach the Earth’s surface in that area. While global efforts like the Montreal Protocol have led to a decrease in ozone-depleting substances, the Antarctic ozone hole still forms each year due to the long lifespan of these chemicals and the specific atmospheric conditions over the South Pole.
Practice Questions (Solved)
Question 1.
(a) What is ‘Atmosphere’ ?
(b) Explain the composition of the Atmosphere ?
(c) Name different layers of atmosphere. Describe the important characteristics of each layer.
Ans:
(a) The ‘Atmosphere’ is the blanket of gases and aerosols that surrounds the Earth, held in place by gravity. It extends from the surface up into space, gradually thinning out with altitude. It plays critical roles in regulating the planet’s temperature, protecting life from harmful solar radiation and space debris, driving weather patterns, and facilitating the water cycle.
(b) The Earth’s atmosphere, a crucial gaseous envelope bound by gravity, is a dynamic blend of various gases and microscopic particles.
Its bulk is composed of permanent gases:
- Nitrogen (N₂), making up about 78%, acts as a stable diluent for oxygen and is fundamental to the global nitrogen cycle, supporting life.
- Oxygen (O₂), at roughly 21%, is indispensable for the respiration of most life forms and for combustion, continuously replenished by plant photosynthesis.
- Argon (Ar), an inert noble gas, comprises about 0.93%.
Beyond these primary constituents, the atmosphere contains minor and variable components:
- Carbon Dioxide (CO₂), though only around 0.04%, is a vital greenhouse gas, regulating the planet’s temperature by trapping heat and being essential for photosynthesis.
- Water Vapor (H₂O) exhibits high variability (0-4%), depending on environmental conditions. It’s the most significant natural greenhouse gas and is critical for the water cycle, cloud formation, and precipitation.
Despite their low concentrations, many, such as methane and ozone, are powerful greenhouse gases or play key roles in atmospheric chemical processes.
Finally, the atmosphere hosts aerosols and particulates, which are microscopic solid and liquid particles. These include dust, pollen, sea salt, volcanic ash, and various pollutants, influencing cloud formation and the way sunlight interacts with the atmosphere (reflection or absorption).
(c)Here’s a breakdown of these layers:
1. Troposphere:
- Description: This is the lowest atmospheric layer, directly adjacent to the Earth’s surface, where all life forms exist.
- Altitude: It typically spans from ground level up to an altitude of approximately 8 to 15 kilometers, with its height varying based on geographic location and seasonal changes.
- Key Features: Nearly all meteorological events, such as cloud formation, precipitation, and storms, take place here. Temperature progressively drops as one ascends through the troposphere. It contains the vast majority of the atmosphere’s water vapor and overall mass.
2. Stratosphere:
- Description: Situated directly above the troposphere, this layer is characterized by its remarkable stability.
- Altitude: It extends from the upper boundary of the troposphere to roughly 50 kilometers above the Earth’s surface.
- Key Features: A critical feature of the stratosphere is the presence of the ozone layer. This layer plays a vital role in absorbing a significant portion of the Sun’s harmful ultraviolet (UV) radiation, converting it into heat and consequently causing temperatures to rise with increasing altitude. This temperature inversion contributes to the layer’s calm and stable conditions, making it suitable for high-altitude aircraft.
3. Mesosphere:
- Description: This layer acts as a protective shield against incoming space debris.
- Altitude: It stretches from about 50 kilometers to approximately 85 kilometers above the Earth.
- Key Features: Temperatures in the mesosphere plummet with increasing altitude, reaching the lowest recorded temperatures within the entire atmosphere. This is the region where most meteors, upon entering Earth’s atmosphere, encounter sufficient friction to ignite and burn up, appearing as “shooting stars.”
4. Thermosphere:
- Description: An expansive and highly energetic layer, the thermosphere is influenced significantly by solar activity.
- Altitude: It begins at about 85 kilometers and can extend upwards to around 1,000 kilometers, with its upper limit fluctuating based on solar radiation levels.
- Key Features: Despite containing extremely tenuous air, temperatures in the thermosphere can soar dramatically due to the absorption of high-energy solar X-rays and ultraviolet radiation. This layer is home to the stunning auroras (the Northern and Southern Lights), which occur when charged solar particles interact with atmospheric gases. It also encompasses the ionosphere, a region of ionized particles crucial for reflecting radio waves, facilitating global communication. The International Space Station (ISS) maintains its orbit within this layer.
Question 2.
What is the significance of solid particles in the atmosphere ?
Ans:
Solid particles in the atmosphere, often referred to as aerosols (when suspended in gas), play a surprisingly significant and multifaceted role in Earth’s systems, impacting everything from weather to climate and even human health. Here’s a brief overview:
- Cloud Formation (Condensation Nuclei): This is perhaps their most vital role. Water vapor in the atmosphere needs a tiny surface to condense upon to form liquid water droplets or ice crystals. Solid particles (like dust, pollen, salt crystals, or smoke) act as these “condensation nuclei” or “ice nuclei.” Without them, clouds and, consequently, precipitation (rain, snow) would be significantly less common, drastically altering the global water cycle.
- Radiation Balance: Solid particles interact with solar radiation.
- Scattering: Light-colored particles (e.g., sulfates from volcanic eruptions or industrial pollution) scatter incoming sunlight back into space, leading to a localized cooling effect on the Earth’s surface.
- Absorption: Dark-colored particles (e.g., black carbon/soot from burning fossil fuels or wildfires) absorb solar radiation, warming the atmosphere where they are present.
- Atmospheric Chemistry: They provide surfaces for various chemical reactions to occur, influencing the composition of the atmosphere. For example, some aerosols can facilitate the formation of acid rain.
- Nutrient Transport: Dust particles, particularly from deserts, can carry essential nutrients (like iron) across vast distances, fertilizing remote oceans and land ecosystems (e.g., Saharan dust fertilizing the Amazon rainforest).
- Visibility: A high concentration of solid particles can significantly reduce visibility, leading to haze and smog, which is a major concern for air quality in urban and industrial areas.
Question 3.
What is the significance of Ozone and what are the effects of its depletion ?
Ans:
Ozone (O3) is a gas that exists in two main layers of the Earth’s atmosphere, and its significance varies greatly depending on its location.
Significance of Ozone:
- Stratospheric Ozone (Good Ozone): This is the ozone layer, located in the stratosphere (15 to 35 kilometers above Earth’s surface). It is incredibly significant because it acts as a natural shield, absorbing most of the Sun’s harmful ultraviolet (UV) radiation, particularly UV-B and UV-C rays. These types of UV radiation are highly damaging to living organisms. By absorbing them, the ozone layer protects:
- Human Health: Reduces the risk of skin cancers (melanoma, basal cell, and squamous cell carcinoma), cataracts, and suppression of the immune system.
- Ecosystems: Protects plants from reduced growth, altered physiological processes (like photosynthesis), and changes in biodiversity. It also safeguards aquatic ecosystems, particularly phytoplankton, which are the base of the marine food chain.
- Materials: Prevents photodegradation of various materials, including polymers and biopolymers, extending their outdoor lifespan.
- Tropospheric Ozone (Bad Ozone): This is ground-level ozone, a harmful air pollutant formed from reactions between pollutants emitted by vehicles, power plants, and industrial facilities in the presence of sunlight. While it’s the same chemical compound, its presence at ground level is detrimental to human health and the environment, causing respiratory problems, damaging crops, and contributing to smog.
Effects of Ozone Depletion:
Ozone depletion refers to the thinning of the stratospheric ozone layer, primarily due to human-made chemicals known as Ozone Depleting Substances (ODS), such as chlorofluorocarbons (CFCs) and halons. The major effects of this depletion are:
- Increased UV Radiation Reaching Earth’s Surface: With less ozone to absorb them, more harmful UV-B and some UV-C rays penetrate the atmosphere.
- Harmful Effects on Human Health:
- Skin Cancers: A significant increase in the incidence of all types of skin cancer, including the more deadly melanoma.
- Eye Damage: Increased risk of cataracts (clouding of the eye’s lens), leading to vision impairment and potential blindness, as well as photokeratitis (“snow blindness”).
- Weakened Immune System: Suppression of the body’s immune response, making individuals more susceptible to infectious diseases.
- Premature Aging of Skin: Increased exposure to UV radiation accelerates skin aging.
- Damage to Ecosystems:
- Terrestrial Plants: Reduced plant growth, impaired photosynthesis, and alterations in plant development and flowering cycles, impacting agricultural yields and natural ecosystems.
- Aquatic Ecosystems: Harm to phytoplankton, which are vital for marine food webs. This can lead to a decline in fish populations and disrupt the entire oceanic food chain. Damage to the early developmental stages of fish, crabs, and amphibians.
- Biodiversity: Changes in species composition and overall ecological balance.
- Impact on Materials: Acceleration of the degradation of synthetic polymers, paints, and other outdoor materials, reducing their lifespan and usefulness.
- Changes in Biogeochemical Cycles: Increased UV-B can alter biogeochemical cycles, potentially affecting the sources and sinks of greenhouse gases and indirectly contributing to climate change.
Question 4.
Define the following
(a) Ozone hole
(b) Tropopause
(c) Global warming
(d) Greenhouse effect
(e) Stratosphere
(f) Troposphere
(g) CFC’s
(h) Mesosphere
(i) Exosphere
(j) Thermosphere
Ans:
Atmospheric & Environmental Definitions
- (a) Ozone Hole: A pronounced, seasonal thinning of the stratospheric ozone layer, predominantly observed over Antarctica, resulting from the interaction of man-made chemicals with specific polar atmospheric conditions.
- (b) Tropopause: The boundary zone separating the troposphere from the stratosphere, identifiable by a distinct shift where the normal decrease in temperature with altitude significantly lessens or reverses.
- (c) Global Warming: The sustained rise in Earth’s average surface temperature, largely a consequence of human-generated greenhouse gases trapping excessive heat within the atmosphere.
- (d) Greenhouse Effect: A vital natural process where atmospheric gases absorb and re-radiate infrared energy, thereby warming the Earth’s surface; its amplification, driven by human activity, fuels global warming.
- (e) Stratosphere: The atmospheric layer situated above the troposphere, distinguished by a temperature increase with elevation, primarily due to the ozone layer’s absorption of ultraviolet (UV) radiation.
- (f) Troposphere: Earth’s lowest atmospheric layer, where all weather phenomena unfold, characterized by temperatures that generally cool as altitude increases.
- (g) CFCs (Chlorofluorocarbons): Human-made chemical compounds, formerly common in refrigeration and aerosols, now recognized for their potent capacity to destroy stratospheric ozone and amplify the greenhouse effect.
- (h) Mesosphere: The atmospheric layer residing above the stratosphere, noted for its decreasing temperatures with height and serving as the region where most incoming meteors disintegrate.
- (i) Exosphere: The Earth’s outermost and most tenuous atmospheric layer, gradually transitioning into the vacuum of space, where gas molecules are exceedingly sparse and rarely collide.
- (j) Thermosphere: The atmospheric layer positioned above the mesosphere, marked by a dramatic increase in temperature with altitude due to the absorption of high-energy solar radiation.
Question 5.
What is atmospheric pressure ?
OR
Is atmospheric pressure the same in every place on the surface of the Earth ?
Ans:
Atmospheric pressure, fundamentally, is the downward force exerted by the entire column of air stretching from Earth’s surface into the vacuum of space, bearing down on a given area. It’s akin to the immense weight of the overlying atmosphere pressing upon everything beneath it.
However, this pervasive pressure isn’t static; it undergoes considerable fluctuations influenced by several critical environmental parameters:
- Altitude’s Influence: Elevation stands as the paramount determinant. Ascending to greater heights, such as scaling a mountain, means the atmospheric column above is progressively shorter and less dense. This diminished volume of overhead air translates directly to a reduction in atmospheric pressure. This phenomenon is why breathing becomes increasingly challenging at high altitudes – there’s less pressure to facilitate oxygen transfer into the lungs. Conversely, at sea level, where the air column is at its maximum extent and density, atmospheric pressure is at its highest.
- Temperature’s Role: The thermal state of air significantly impacts its pressure. Warmer air is inherently lighter and less dense than its cooler counterpart. As air molecules gain thermal energy, they accelerate, spread out, and consequently exert less pressure. Therefore, warm air masses are typically associated with lower pressure. This correlation underpins the common meteorological observation that low-pressure systems often herald warm, overcast, and potentially stormy weather, while high-pressure systems are frequently linked to cold, clear, and stable atmospheric conditions.
- Humidity’s Peculiarity: Surprisingly, air laden with water vapor (humid air) is actually lighter than dry air at identical temperatures and pressures. This is because water molecules (H2O) possess a lower molecular mass than the average molecular mass of dry air (which is predominantly nitrogen (N2) and oxygen (O2)). Consequently, an increase in atmospheric humidity tends to induce a slight decrease in overall atmospheric pressure.
- Dynamic Weather Systems (High and Low Pressure): Large-scale atmospheric circulation patterns give rise to mobile high-pressure and low-pressure systems that continuously traverse the Earth’s surface.
- High-pressure systems are characterized by descending air that diverges outwards.
- Low-pressure systems, conversely, are regions where air ascends and converges inwards. This rising air cools and expands, frequently fostering cloud formation and precipitation, alongside comparatively reduced pressure.
- Coriolis Effect and Planetary Rotation: The Earth’s rotational motion, in concert with the Coriolis effect (a force arising from the planet’s rotation), exerts significant control over the trajectory of expansive air masses. This intricate interplay is instrumental in the genesis and migration of these pressure systems, which, in turn, orchestrates localized and broader regional variations in atmospheric pressure across the globe.
Question 6.
Why does the atmosphere thin out at higher levels ?
Ans:
The atmosphere thins out at higher levels primarily due to gravity and the compressibility of gases.
Here’s a detailed explanation:
- Gravity’s Pull: This means that the vast majority of air molecules are concentrated closer to the planet’s surface. Think of it like a stack of pillows: the pillows at the bottom are compressed by the weight of all the pillows above them, making the stack denser at the bottom. The same principle applies to air molecules.
- Weight of Overlying Air (Atmospheric Pressure): Atmospheric pressure is essentially the weight of the column of air above a given point. At sea level, you have the entire column of the atmosphere pressing down on you, resulting in the highest atmospheric pressure and therefore the densest air.With less weight pressing down, the air molecules are less compressed and can spread out more, leading to lower pressure and consequently, lower density (thinner air).
- Compressibility of Gases: Unlike liquids, gases are highly compressible. This means their volume can change significantly with changes in pressure. At lower altitudes, the immense weight of the air above compresses the air molecules into a smaller volume, making the air denser. At higher altitudes, with less pressure, the gas molecules expand and occupy a larger volume, making the air less dense or “thinner.”
- Molecular Spacing: Because of these factors, the air molecules at higher altitudes are much farther apart from each other compared to those at lower altitudes. This wider spacing means there are fewer air molecules, and thus fewer oxygen molecules, in a given volume of air as you go higher up. This is why mountaineers require supplemental oxygen at extreme altitudes.
Question 7.
What are the properties of the Troposphere and Ionosphere?
Ans:
The Troposphere: Earth’s Dynamic Weather Engine
Spanning from the Earth’s surface up to 8-15 km, its height fluctuates with latitude and season. Uniquely, temperature decreases with altitude here because the ground warms the air, which then cools as it rises.
It’s the densest layer, holding 75-80% of the atmosphere’s mass and virtually all its water vapor. This high density, combined with the temperature gradient, fuels convection currents, leading to clouds, precipitation, and winds. It’s a turbulent, dynamic zone providing the breathable air essential for life and regulating our climate through the natural greenhouse effect.
The Ionosphere: Earth’s Electrified Upper Shield
The ionosphere isn’t a standalone layer but an ionized region spanning roughly 60-1000 km, encompassing parts of the mesosphere, thermosphere, and exosphere. Its defining feature is the presence of free electrons and ions, created when high-energy solar and cosmic radiation strips electrons from neutral atoms.
This ionization leads to a layered structure (D, E, F1, F2) that varies dramatically between day and night. Daytime sees more pronounced layers, while nighttime causes some to disappear or merge as ions recombine. Crucially, these charged particles enable radio wave reflection, vital for long-distance communication, allowing signals to “bounce” around the globe.
Despite extremely high kinetic temperatures, the sparse particle density means it wouldn’t feel hot. It’s a highly dynamic region, constantly influenced by solar activity, which also causes the stunning auroras when charged particles interact with its gases. Its varying electron density can also affect GNSS/GPS signals, causing minor delays.
Question 8.
Give reasons for the following :
- The Earth does not experience extremes of temperature as in other planets.
- The amount of water vapour in the atmosphere varies from place to place.
- Solid particles play an important role in the atmosphere.
- In the troposphere, the temperature decreases with height.
- The stratosphere is crucial to life on Earth.
- The ionosphere (thermosphere) is suited to long distance communication.
- As a jet plane flies high in the sky, it leaves a white trail behind.
- The exosphere allows the gas molecules to easily escape into space.
- Dust particles play a significant role in the atmosphere.
Ans:
Earth’s Stable Climate
Earth maintains remarkably steady temperatures due to its moderate greenhouse effect, which traps just enough heat. Our planet’s vast oceans and atmosphere also act as a global thermostat, regulating and redistributing heat through currents and winds, preventing the extreme temperature swings seen on other celestial bodies.
Variable Water Vapor
The amount of water vapor in the atmosphere changes geographically, influenced by higher evaporation rates over warm water bodies, air’s capacity to hold moisture based on temperature, and large-scale atmospheric circulation that transports this moisture, creating diverse humidity levels.
Solid Particles’ Atmospheric Contribution
Tiny solid particles like dust, pollen, and soot are vital for cloud formation, acting as surfaces for water vapor to condense on. They also affect Earth’s energy balance by scattering and absorbing solar radiation, influencing visibility and atmospheric temperatures.
Tropospheric Temperature Decline
In the troposphere, air temperature drops with increasing altitude because the Earth’s surface is the primary heat source.
Stratosphere’s Vital Role
Without this shield, high levels of UV rays would reach Earth, causing severe harm to living organisms and making life as we know it impossible.
Ionosphere and Long-Distance Communication
The ionosphere, part of the thermosphere, enables long-distance radio communication because its ionized layers (electrically charged gases) can reflect radio waves back to Earth. This allows signals to travel beyond the line of sight and over the planet’s curvature.
Jet Plane Contrails
Jet planes leave white contrails when their hot, moist exhaust mixes with the extremely cold, low-pressure air at high altitudes. The water vapor in the exhaust rapidly condenses and freezes into visible ice crystals, forming a cloud-like streak.
Exosphere: Gateway to Space
The exosphere is Earth’s outermost atmospheric layer, characterized by extremely low density and weak gravity. Gas molecules are so sparse that they can gain enough energy to escape Earth’s gravitational pull and drift into space, marking the atmosphere’s transition to the cosmos.
Dust Particles: Atmospheric Architects
Dust particles are significant in the atmosphere as essential condensation nuclei for forming clouds and fog. They also scatter incoming sunlight, creating colorful sunrises and sunsets, and can absorb solar radiation, impacting local temperatures and visibility.
Question 9.
‘Atmosphere is the most dynamic element in the Environment’. Discuss.
Ans:
The atmosphere is arguably the most dynamic element in the environment because it is constantly in motion and undergoes continuous physical and chemical transformations. This dynamism is driven primarily by solar energy and Earth’s rotation, resulting in a constant interplay of forces and phenomena:
- Continuous Movement: Air masses are always moving, from local breezes to global circulation patterns like trade winds and jet streams.
- Weather Phenomena: All weather events, from rain and snow to thunderstorms and hurricanes, are direct manifestations of the atmosphere’s dynamic nature. These phenomena arise from complex interactions of temperature, pressure, humidity, and air currents.
- Energy Transfer: The atmosphere is a crucial medium for transferring solar energy around the planet. It absorbs, reflects, and re-radiates heat, creating the conditions for life and influencing oceanic currents and land temperatures.
- Compositional Changes: While major gases like nitrogen and oxygen remain relatively stable, trace gases and aerosols are constantly changing due to natural processes (volcanic eruptions, wildfires) and human activities (emissions). These changes, even minor ones, can have significant impacts, as seen with the ozone layer and greenhouse gases.
- Interaction with Other Spheres: The atmosphere is in continuous interaction with the hydrosphere (water cycle), lithosphere (dust, volcanic gases), and biosphere (plant respiration, human emissions), making it a central component of interconnected Earth systems.
Question 10.
‘The atmosphere acts as a blanket or a glasshouse’. Discuss.
Ans:
The Earth’s atmosphere acts much like a thermal blanket or a glasshouse, a perfect analogy for understanding the greenhouse effect.
Think of a warm blanket: it doesn’t create heat, but it traps the warmth radiating from your body, preventing it from escaping. Similarly, specific gases in our atmosphere—like carbon dioxide, methane, and water vapor—absorb the heat (infrared radiation) that Earth radiates after being warmed by the sun. They then re-emit this heat back towards the surface, effectively trapping warmth within the lower atmosphere. Without this natural “blanket,” Earth’s average temperature would plummet to a freezing -18°C (0°F), making life as we know it impossible.
A glasshouse (or greenhouse) works on the same principle. Its glass panes let sunlight in to warm the interior, but they block much of the outgoing heat, keeping the inside significantly warmer than the outside. In the same way, atmospheric greenhouse gases allow the sun’s shortwave radiation to reach Earth, but then prevent the longer-wavelength heat from easily escaping back into space.
These analogies powerfully illustrate why Earth is naturally habitable. They also help us grasp global warming: if we increase the concentration of these heat-trarapping gases, it’s like making the blanket thicker or the glasshouse panes denser, leading to an amplified warming effect on our planet.