Matter, defined as anything with mass and volume, is made of tiny particles (molecules/atoms). The Kinetic Theory explains their behavior: particles are always moving (kinetic energy), have spaces between them (intermolecular spaces), and attract each other (intermolecular forces, stronger when closer).
These principles define the three states:
- Solids: Densely packed particles, strong forces, fixed positions. Result: definite shape/volume, rigid, incompressible.
- Liquids: Looser particles, weaker forces, free movement within boundaries. Result: definite volume, takes container shape, slightly compressible.
- Gases: Widely spaced particles, negligible forces, random motion. Result: no definite shape/volume, highly compressible.
The chapter also covers state changes (melting, boiling, etc.) due to temperature/pressure altering particle energy and spacing, defining terms like melting and boiling points.
Test Yourself
A. Objective Questions
1. Write true or false for each statement
(a) The temperature of a substance remains unaffected during its change of state.
Ans: True.
(b) Ice melts at 100°C.
Ans: False. The ice melts at 0° by absorption of heat.
(c) Water at 100°C has more heat than the steam at 100°C.
Ans: False.
(d) Evaporation of a liquid causes cooling.
Ans: True.
(e) Water evaporates only at 100°C.
Ans: False.
(f) Boiling takes place at all temperatures.
Ans: False.
(g) Evaporation takes place over the entire mass of the liquid.
Ans: False.
(h) The process of a gas converting directly into solid is called vaporization.
Ans: False.
The process of a liquid converting directly into gas is called vaporization.
(i) At high altitudes water boils above 100° C.
Ans: False.
(j) The melting point of ice is 0°C.
Ans: True.
2. Fill in the blanks
(a) Evaporation takes place at _______temperatures.
Ans: all
(b)____________process is just reverse of melting
Ans: Freezing
(c) _________ is a process that involves direct conversion of a solid into its vapour on heating.
Ans: Sublimation
(d) The temperature at which a solid converts into a liquid is called its ___________
Ans: melting point.
(e) The smallest unit of matter that exists freely in nature is called a __________
Ans: molecule.
(f) Molecules of a substance are always in a state of ____________ and so they possess _____________.
Ans: motion,kinetic energy
(g) Intermolecular space is maximum in___________and the least in ____________.
Ans: gases, less in liquids ,solids
(h) Intermolecular force of attraction is maximum in ____________.
Ans: solids, less in liquids and the least in gases
3. Match the following
4. Select the correct alternative
(a) The inter-molecular force is maximum in
- solids
- gases
- liquids
- none of the above
Ans:solids
(b) The inter-molecular space is maximum in
- liquids
- solids
- gases
- none of the above
Ans:gases
(c) The molecules can move freely anywhere in
- gases
- liquids
- solids
- none of the above
Ans:gases
(d) The molecules move only within the boundary of
- liquids
- gases
- solids
- none of the above
Ans: liquids
(e) The temperature at which a liquid gets converted into its vapour state is called its
- melting point
- boiling point
- dewpoint
- freezing point.
Ans:boiling point
(f) Rapid conversion of water into steam is an example of
- evaporation
- freezing
- melting
- vapourization.
Ans:vapourization
(g) Evaporation takes place from the
- surface of liquid
- throughout the liquid
- mid-portion of the liquid
- bottom of liquid.
Ans:surface of liquid
(h) Boiling takes place from the
- the surface of the liquid
- throughout the liquid
- mid-portion of liquid
- none of the above.
Ans:throughout the liquid
Short/Long Answer Questions
1)Define the term matter. What is it composed of ?
Ans:Matter, at its core, encompasses everything tangible around us, whether it’s the invisible air we inhale, the solid ground beneath our feet, or the warmth of a cup of coffee. This incredible variety in the physical world stems from a fundamental shared characteristic: all matter is constructed from minuscule units known as molecules. These molecules, in turn, are themselves assemblies of even smaller constituents called atoms. The specific way these atoms and molecules are arranged, along with the types of atoms involved, dictates the distinct properties that define each substance.
2)State three properties of molecules of a matter.
Ans:Particles of matter are tiny, separated by empty spaces, and are in constant, random motion, possessing kinetic energy. The extent of this motion and the spacing between them define the different states of matter.
3)What do you mean by the inter-molecular spaces ? How do they vary in different states of matter ?
Ans:Intermolecular spaces, the gaps between molecules, determine a substance’s state. Solids have tiny gaps, giving them a fixed shape and volume. Liquids possess larger gaps, allowing them to flow and take the shape of their container. Gases exhibit the largest gaps, making them easily expandable and compressible. A good example is dissolving salt in water; the salt fits into the water’s existing molecular spaces, so the overall volume doesn’t change much.
4)What is meant by the inter-molecular forces of attraction ?
Ans:Intermolecular forces (IMFs) dictate a substance’s physical state. Strongest in solids, IMFs hold molecules rigidly, giving them a fixed shape and volume and making them incompressible. In liquids, moderate IMFs allow molecules to flow while remaining close, resulting in a definite volume but an adaptable shape, with minimal compressibility. Gases exhibit the weakest IMFs, allowing molecules to disperse widely, leading to no definite shape or volume and high compressibility
5)Which of the following are correct ?
(a) Solids have definite shape and definite volume.
Ans: True.
Reason As the molecules here have negligible intermolecular distance between them and have maximum intermolecular force of attraction.
(b) Liquids have definite volume but do not have definite shape.
Ans: True.
(c) Gases have definite volume but no definite shape.
Ans: False.
Correct Gases have neither definite volume nor a definite shape.
(d) Liquids have definite shape and definite volume.
Ans:False.
Correct Liquids have a definite volume but not definite shape.
6)Discuss the three states of matter: solid, liquids and gas on the basis of molecular models.
Ans:Solids
In solids, molecules are densely packed with minimal space between them, resulting in strong intermolecular forces. This high cohesion restricts molecular movement, allowing only vibrations around fixed positions. Consequently, solids maintain a definite shape and volume.
Liquids :
In the liquid state, molecules possess greater kinetic energy than their counterparts in solids. This heightened energy grants them increased mobility, allowing for freer movement, though they remain in relatively close proximity to one another, unlike the widely dispersed molecules in gases. The intermolecular forces present in liquids, while diminished compared to those in solids, retain sufficient strength to maintain molecular cohesion, thereby imparting a definite volume to the liquid. Nevertheless, these forces lack the rigidity to anchor molecules in fixed positions, which explains why liquids readily flow and effortlessly adapt to the contours of their containing vessel
Gases :
In gases, the molecules are quite spread out, meaning there’s a significant amount of space between them. This large intermolecular distance leads to very weak forces of attraction between individual molecules. Because these molecules aren’t held together by strong bonds, they’re free to move around independently and rapidly. This constant, unconstrained motion is why gases don’t possess a fixed shape; they’ll simply expand to fill whatever container they’re in. Similarly, they lack a definite volume, as their molecules will disperse to occupy the entire available space.
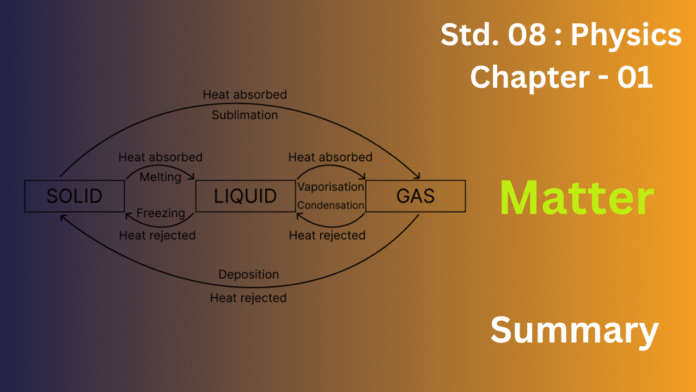
7)What do you mean by the change of state ? Write the flow chart showing the complete cycle of change of state.
Ans:Change of State: This refers to a substance transforming from one form to another, occurring at a constant temperature through the gain or loss of heat.
Complete Cycle: When a solid is heated, it melts into a liquid. Further heating converts the liquid into a vapor. Conversely, cooling vapors causes them to condense back into a liquid, and continued cooling freezes the liquid into a solid. It’s also worth noting that some solids can directly turn into vapors upon heating (sublimation), and these vapors can solidify directly upon cooling (deposition).
This cycle can be shown by diagram
8)Differentiate between melting point and boiling point, giving at least one example of each.
Ans:Melting point is when a solid turns into a liquid, with the absorbed energy breaking bonds rather than raising temperature. Think of ice at 0°C. The boiling point is when a liquid becomes a gas, occurring when its vapor pressure matches the surrounding air pressure. Water, for instance, boils at 100°C.
9)Describe the process of condensation and sublimation with examples.
Ans:Condensation is when a gas changes into a liquid, often due to cooling or pressure. Think of dew on grass or a steamy bathroom mirror.
Sublimation is the direct shift from a solid to a gas, bypassing the liquid phase. Examples include dry ice vaporizing or snow seemingly disappearing without melting.
10)Explain the term melting and melting point.
Ans:Melting occurs when a solid absorbs sufficient heat, enabling its particles to move freely and transition into a liquid state. The melting point, a distinct property for pure substances, is the precise temperature at which this transformation takes place, allowing both solid and liquid phases to coexist
11)Describe an experiment to demonstrate that a substance absorbs heat during melting without change in its temperature.
Ans:The melting point of wax was determined to be 55°C. During the experiment, the wax’s temperature steadily increased until it reached 55°C, at which point it began to melt and remained at this temperature for about five minutes. This constant temperature during the phase change indicates that the heat supplied was absorbed as latent heat, converting the wax from solid to liquid without a rise in temperature. Once all the wax melted, its temperature resumed rising.
12)Explain the terms vaporization and boiling point.
Ans:Vaporization is the process where a liquid transforms into vapor (gas) when heated. Initially, the liquid’s temperature increases. However, once it reaches a specific temperature, the temperature remains constant even with continued heating. This sustained heat energy is used to convert all the liquid molecules into vapor, rather than increasing the temperature further.
This constant temperature at which a liquid begins to change into vapor is known as its Boiling Point.
13)A liquid can change into vapour state
(a) at a fixed temperature, and
(b) at all temperatures
Name the processes involved in two cases.
Ans:a) Boiling is when a liquid rapidly turns into vapor at a specific temperature, its boiling point, with bubbles forming throughout. This requires absorbing latent heat.
(b) Evaporation is the slower process where a liquid changes to vapor only at its surface and at any temperature below its boiling point.
14)Some ice is taken in a beaker and its temperature is recorded after each one minute. The observations are listed below
From the above observations what conclusion do you draw about the melting point of ice ?
Ans:When ice at 0∘C melts, the supplied heat, known as latent heat of fusion, doesn’t raise its temperature. Instead, this energy breaks the ice’s rigid bonds, allowing water molecules to move freely and transition into a liquid. Only after all the ice has melted will the water’s temperature start to increase.
15)Describe an experiment to demonstrate that water absorbs heat during boiling at a constant temperature.
Ans:BOILING POINT OF WATER AT CONSTANT TEMPERATURE:
When heating water, the temperature steadily increases until it reaches 100°C. At this point, even with continuous heat supply, the temperature remains constant. This 100°C is the boiling point of water. The added heat at this stage is not raising the temperature but is instead being used to convert the liquid water into steam (vapor), as individual water molecules gain enough energy to escape into the gaseous phase.
16)State (a) the melting point of ice, and (b) the boiling point of water.
Ans:(a) Melting Point of Ice: Ice turns to water at 0∘C. This temperature remains constant during the phase change as absorbed heat provides the latent heat of fusion.
(b) Boiling Point of Water: Water converts to steam at 100∘C at standard atmospheric pressure. Similar to melting, the temperature holds steady during boiling due to the latent heat of vaporization. The boiling point can vary with atmospheric pressure.
17)What is evaporation ?
Ans:Evaporation is the gentle shift of a liquid into a gas, occurring even when the temperature is below its boiling point. This transformation mainly takes place at the liquid’s surface, where certain molecules absorb enough energy to escape into the surrounding air. Common examples include a wet road drying in the sun or clothes on a line becoming crisp.
18)State three factors which affect the rate of evaporation of a liquid.
Ans:
(i) Surface Area: Imagine spreading out your wet laundry; it dries much faster than if it were left in a crumpled heap. This is because a larger exposed surface allows more liquid molecules to directly interact with the surrounding air, giving them a greater chance to escape as vapor.
(ii) Temperature: Think about a puddle on a hot, sunny day versus a cold, cloudy one. The hot puddle disappears much quicker. This is because higher temperatures provide the liquid molecules with more energy, making it easier for them to break free from the liquid’s surface and transition into a gaseous state.
(iii) Nature of the Liquid: Not all liquids evaporate at the same pace. Liquids like rubbing alcohol, with weaker attractive forces between their molecules, tend to evaporate more readily than, say, water, which has stronger intermolecular bonds. Less energy is needed for these weaker bonds to be overcome, allowing molecules to escape more easily.
(iv) Humidity: The air’s existing moisture content, or humidity, plays a significant role. If the air is already laden with water vapor, like on a muggy day, there’s less “room” for more water molecules to evaporate into it. Conversely, on a dry day, the air can readily absorb more moisture, leading to faster evaporation.
19)Wet clothes dry more quickly on a warm dry day than on a cold humid day. Explain.
Ans:On a warmer day, molecules in liquid water gain more kinetic energy due to the higher ambient temperature. This increased energy allows a greater number of water molecules at the surface to overcome the intermolecular forces holding them in the liquid state and escape into the air as vapor. Consequently, the rate of evaporation accelerates, leading to clothes drying much faster than on a colder day with lower temperatures.
20)Water in a dish evaporates faster than in a bottle. Give a reason.
Ans:Evaporation is fundamentally a surface process. The more liquid molecules directly exposed to the surrounding air, the greater the chance they have to escape into the gaseous state. Therefore, increasing the surface area of a liquid, such as by spreading it out in a wide, shallow dish, accelerates the rate at which it evaporates compared to confining the same volume in a narrow, deep container.
21)Why are volatile liquids such as alcohol and spirit stored in tightly closed bottles ?
Ans:Volatile liquids like alcohol and spirit evaporate readily because the attractive forces between their molecules are quite weak. This minimal attraction means individual molecules can easily break away from the liquid’s surface and turn into a gas, even at typical room temperatures.
To prevent these liquids from quickly disappearing, they are kept in sealed containers. This traps the vapor that forms above the liquid, creating a higher concentration of gaseous molecules. This increased vapor pressure then pushes back on the liquid’s surface, slowing down or “inhibiting” further rapid evaporation.
22)A certain quantity of water is heated from 20°C to 100°C. Its temperature is recorded after each 1 minute. The observations are:
What conclusion do you draw from the above table about the boiling point of water ? Explain.
Ans: When a thermometer reads a steady 100°C while heating water, it signifies that this is the boiling point of water. At this stage, despite continuous heat input, the temperature doesn’t rise further. Instead, all the supplied energy is being utilized to transform the liquid water into water vapor (steam), molecule by molecule, until all the water has completely boiled away.
23)Why is cooling produced on evaporation of a liquid ?
Ans: When a liquid like alcohol evaporates from your skin, it absorbs energy from your body, making the area feel cool. This is the same reason sweating helps cool us down: as sweat evaporates, it takes heat with it, regulating our body temperature.
24)Explain with an example to demonstrate that when a liquid
evaporates, it takes heat from its surroundings.
Ans:When spirit is applied to a thermometer bulb, the reading drops because the spirit rapidly evaporates. This evaporation is a cooling process; the liquid absorbs heat energy from its immediate surroundings (in this case, the thermometer bulb) to change into a gas. This loss of heat from the bulb causes the thermometer’s temperature reading to fall.
25)Give two applications of evaporation.
Ans:(i) Sprinkling water on scorching summer roads provides immediate relief because as the water turns into vapor, it draws warmth directly from the road surface and the air around it. This heat absorption during evaporation leads to a noticeable cooling effect, making the environment much more comfortable.
(ii) That delightful coolness you feel after a summer downpour is entirely due to the water evaporating from your skin. As the water changes phase from liquid to gas, it pulls thermal energy away from your body, leaving you with that wonderfully refreshing sensation.
26)Explain why on hot summer days water remains cool in earthen pots.
Ans:Earthen pots cool water through evaporative cooling. Water seeps through tiny pores in the pot, and as it evaporates from the outer surface, it draws the necessary latent heat from the water remaining inside, thereby lowering its temperature.
27)A patient suffering from high fever is advised to put wet clot strips on his forehead. Why ?
Ans:To cool someone with a fever, wet cloths are effective due to several principles:
- Evaporation: Water on the cloth absorbs heat from the body to turn into vapor, taking warmth with it. This is a primary cooling mechanism.
- Direct Heat Transfer: The cooler cloth itself directly draws heat from the hotter skin upon contact.
- Circulation: Placing cloths on areas like the forehead, where blood vessels are close to the surface, helps cool the blood. This cooled blood then circulates, aiding in lowering the overall body temperature.
- Continuous Application: Regularly replacing the warming cloths with fresh, cool ones ensures the cooling effect is maintained.
In essence, this method utilizes the cooling power of evaporation and direct heat transfer, amplified by blood circulation, to help reduce a fever.
28)What do you mean by sublimation ? Explain with an example.
Ans:SUBLIMATION : Sublimation is a unique physical process where a substance changes directly from a solid to a gaseous state without passing through a liquid phase. This occurs when the substance’s vapor pressure is high enough at a temperature below its melting point.
Example: A common example is dry ice, which is solid carbon dioxide (CO2). When exposed to room temperature, dry ice doesn’t melt into a liquid; instead, it directly transforms into carbon dioxide gas, creating a visible “fog” often used for special effects. Similarly, naphthalene mothballs also sublime over time, slowly releasing their characteristic odor as they turn from solid to gas.
29)Why does the size of naphthalene balls decrease when left open ?
Ans:Naphthalene balls shrink and eventually disappear when left open due to a process called sublimation. This means that the solid naphthalene directly turns into a gas, skipping the liquid phase entirely. As the solid converts into gas and disperses into the air, the size of the naphthalene ball gradually decreases.
30)Describe an experiment to demonstrate the process of sublimation.
Ans:Experiment: This experiment beautifully illustrates sublimation. When heated, solid ammonium chloride transforms directly into a gas, bypassing the liquid phase entirely. As these gaseous vapors encounter the cooler funnel, they directly revert to solid ammonium chloride, skipping the liquid state once more.